Professional Documents
Culture Documents
Experiment 3. Osmosis Demonstration Using A Biological Membrane
Experiment 3. Osmosis Demonstration Using A Biological Membrane
Uploaded by
Anonymous A8vAWcHBOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Experiment 3. Osmosis Demonstration Using A Biological Membrane
Experiment 3. Osmosis Demonstration Using A Biological Membrane
Uploaded by
Anonymous A8vAWcHBCopyright:
Available Formats
Experiment 3.
Osmosis demonstration using a biological membrane
Each group will use a shelless chicken egg. The shell has been dissolved away leaving the shell membrane and the
rest of the egg intact. The membrane selectively permits some kinds of molecules to pass through while preventing
others from doing so.
Procedure:
1. Carefully (fragile!) remove the shelless egg from the container and gently pat it dry to remove any excess surface
water. Immediately weigh the egg, record the weight and place it into the beaker containing corn syrup.
2. Repeat the drying and weighing process after 15, 30, 45 and 60 minutes in the corn syrup. Record the results of
each weighing in Table 3 (below).
Note: When the egg is first placed in the corn syrup, half of the egg will not be submerged. GENTLY roll the egg with the plastic spoon to make
certain that all of the membrane is in contact with the syrup.
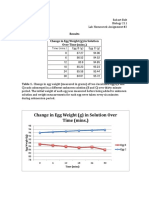
Table 3 Weights for Shelless Chicken Egg
Time Intervals (min.)
0 15 30 45
Initial Weight (g) 98 98 98 98
(Does not change)
New Weight (g) ----- 90 84 79
Net Gain / Loss(g) ----- -8 -14 -19
% Change in mass* ----- -8.16% -14.28% -19.38%
*Percent change in mass = (new wt initial wt) / initial wt X 100
Questions:
1. Was there a change in the weight of the egg? Briefly explain what happened and why using the terms
hypertonic, hypotonic and osmosis.
2. The diagram below represents the egg and the beaker in which it was placed. Label the solution inside the
egg and the solution inside the beaker as hypertonic or hypotonic. Using an arrow, indicate the
direction in which water flowed: into, or out of the cell (egg).
Beaker
Egg
You might also like
- Lab Food Analaysis-FatDocument9 pagesLab Food Analaysis-FatSHAFIKANOR366160% (5)
- FST 209 - Chapter 2Document26 pagesFST 209 - Chapter 2Muhd Syahmi100% (1)
- Food Engg LE 4 Final 1Document21 pagesFood Engg LE 4 Final 1MonicaFrancesSalvadorNo ratings yet
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- Nutri Lab ReviewerDocument53 pagesNutri Lab ReviewerRaquel MonsalveNo ratings yet
- SBK Lab Report 4Document12 pagesSBK Lab Report 4Nisha Lauren VishvanathNo ratings yet
- BiologyDocument6 pagesBiologyAnonymous A8vAWcHBNo ratings yet
- Part II. Osmosis: Osmosis Demonstration Using An Artificial MembraneDocument2 pagesPart II. Osmosis: Osmosis Demonstration Using An Artificial MembraneAnonymous A8vAWcHBNo ratings yet
- Results Change in Egg Weight (G) in Solution Over Time (Mins.)Document2 pagesResults Change in Egg Weight (G) in Solution Over Time (Mins.)Roberto BeltNo ratings yet
- NEw Polarimetry SupplementDocument2 pagesNEw Polarimetry Supplementraygius77No ratings yet
- FC Egg White ReportDocument13 pagesFC Egg White ReportGrace OngNo ratings yet
- Analises FatDocument1 pageAnalises FatTeresa FreitasNo ratings yet
- Mbpracticas LabDocument6 pagesMbpracticas LabRosa VelásquezNo ratings yet
- PCE - Chapter 4 - MASS BALANCE-47-73 PDFDocument27 pagesPCE - Chapter 4 - MASS BALANCE-47-73 PDFSchool Of Energy UTPNo ratings yet
- Lab Report 1 Final LabDocument9 pagesLab Report 1 Final LabKristel May SomeraNo ratings yet
- Summerscience JordangabrielsamuelDocument5 pagesSummerscience Jordangabrielsamuelapi-289533905No ratings yet
- Acetone ExperimentDocument6 pagesAcetone ExperimentnasrishamiehNo ratings yet
- "Understanding The Concepts of Egg Osmosis ": Zoology LaboratoryDocument7 pages"Understanding The Concepts of Egg Osmosis ": Zoology LaboratoryAlegado, AldrichNo ratings yet
- Effect of Tribulus Terrestris Extract On Semen Quality and Serum Total Cholesterol Content in White Plymouth Rock-Mini CocksDocument8 pagesEffect of Tribulus Terrestris Extract On Semen Quality and Serum Total Cholesterol Content in White Plymouth Rock-Mini CocksyigalbyNo ratings yet
- FST306 Laboratory Report 2Document8 pagesFST306 Laboratory Report 2Nisa AzamNo ratings yet
- Ves Zte Seg Vala Miez IsDocument10 pagesVes Zte Seg Vala Miez IsSzekrényi KrisztaNo ratings yet
- Técnica de Medición de Biomasa (Peso Seco, Conteo Directo)Document6 pagesTécnica de Medición de Biomasa (Peso Seco, Conteo Directo)JUAN DAVID GAMBOA MORENONo ratings yet
- Menu Planning SimplifiedDocument5 pagesMenu Planning Simplifiedshannon c. lewisNo ratings yet
- Total Starch Colorimetric MethodDocument8 pagesTotal Starch Colorimetric MethodSOCKYNo ratings yet
- Biological Psychology - Work AssignmentDocument8 pagesBiological Psychology - Work AssignmentM.FrostNo ratings yet
- Cheese Lab ReportDocument7 pagesCheese Lab Reportapi-352673659No ratings yet
- Controling Air Bubbles in FlanDocument5 pagesControling Air Bubbles in FlanFungiipNo ratings yet
- Food Chemistry I Laboratory: TC Haliç University Faculty of Health Sciences Department of Nutrition and DieteticsDocument29 pagesFood Chemistry I Laboratory: TC Haliç University Faculty of Health Sciences Department of Nutrition and Dieteticsmemebeauty220No ratings yet
- Reducing The Risks of Surface Prion ContaminationDocument2 pagesReducing The Risks of Surface Prion ContaminationSol AnayaNo ratings yet
- Poster Final For ColloquimDocument11 pagesPoster Final For ColloquimLessie Mae Dela Cruz-MataNo ratings yet
- Extrusado TruchaDocument14 pagesExtrusado TruchaAlex FloresNo ratings yet
- Calculating DietsDocument7 pagesCalculating DietsJEWEL DEEN VILLARMENTE OQUIANANo ratings yet
- Nutrilab Meal Plan-GiDocument12 pagesNutrilab Meal Plan-GiALLAN TROI RAMOSNo ratings yet
- Foam FormulationsDocument9 pagesFoam FormulationsRobelyn Eusebio BacalangcoNo ratings yet
- Lab Report Fat AnalysisDocument10 pagesLab Report Fat Analysisnurqistina hanani HananNo ratings yet
- Homogenization and Cream SeperationDocument11 pagesHomogenization and Cream Seperationkenanoz100% (4)
- CFB20703-Food Chem Exp 1 PDFDocument4 pagesCFB20703-Food Chem Exp 1 PDFsitinurhanizaNo ratings yet
- Effect of Homogenizing Pressure and Sterilizing Condition On Quality of Canned High Fat Coconut MilkDocument7 pagesEffect of Homogenizing Pressure and Sterilizing Condition On Quality of Canned High Fat Coconut Milkthaihoangminh93No ratings yet
- Mass BalanceDocument45 pagesMass BalanceDafik A. MasruriNo ratings yet
- Experiment 1 - WeighingDocument3 pagesExperiment 1 - WeighingSiti Kay Sara100% (1)
- Dairy Tech Assignment 1Document13 pagesDairy Tech Assignment 1Mitchelle ChikakaNo ratings yet
- Experiment 1: Basic Laboratory TechniquesDocument8 pagesExperiment 1: Basic Laboratory Techniquesdaffa MadriNo ratings yet
- Exp. 7 Analysis of Milk For The Lipids Carbohydrates and ProteinsDocument5 pagesExp. 7 Analysis of Milk For The Lipids Carbohydrates and ProteinsDoc Zay VillafuerteNo ratings yet
- Sadie Ikeda - Cheese Lab Write UpDocument10 pagesSadie Ikeda - Cheese Lab Write Upapi-342516670No ratings yet
- Telur Dan OlahannyaDocument50 pagesTelur Dan OlahannyaRosa TrihertamawatiNo ratings yet
- Lab Manual Experiment 5Document11 pagesLab Manual Experiment 5ShafikaNo ratings yet
- Turbidimeter LutronDocument13 pagesTurbidimeter LutronYuseth Patio AvilesNo ratings yet
- Bab 03 MassBalance 2009Document39 pagesBab 03 MassBalance 2009sofiapurnamaNo ratings yet
- Ib Potato Osmolarity LabDocument4 pagesIb Potato Osmolarity LabLuesma Fully (STUDENT)No ratings yet
- Lab 2Document6 pagesLab 2Lenoj OlarNo ratings yet
- Chemistry LabDocument3 pagesChemistry LabbakerroeNo ratings yet
- Egg Osmosis FINAL DRAFT RemovedDocument2 pagesEgg Osmosis FINAL DRAFT RemovedSamson Lee Yun ShenNo ratings yet
- Data Collection: 1. Quantitative DataDocument5 pagesData Collection: 1. Quantitative DataNabila Azureen AzisNo ratings yet
- DineshKhadka - Greenwich University UKDocument16 pagesDineshKhadka - Greenwich University UKDinesh KhadkaNo ratings yet
- Telur Dan OlahannyaDocument46 pagesTelur Dan OlahannyaRosa TrihertamawatiNo ratings yet
- Chaper 4 Non-Reactive Multi Units ProcessDocument48 pagesChaper 4 Non-Reactive Multi Units Processجنات الغبيراءNo ratings yet
- Cell Diffusion & Permeability - Basic VersionDocument9 pagesCell Diffusion & Permeability - Basic VersionTeachLABScINo ratings yet
- Instant Pot Family Meals: 60+ Fast, Flavorful Means for the Dinner TableFrom EverandInstant Pot Family Meals: 60+ Fast, Flavorful Means for the Dinner TableRating: 4 out of 5 stars4/5 (2)