Professional Documents
Culture Documents
Acute Pancreatitis: Seminar
Uploaded by
Karen JboOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acute Pancreatitis: Seminar
Uploaded by
Karen JboCopyright:
Available Formats
Seminar
Acute pancreatitis
Paul Georg Lankisch, Minoti Apte, Peter A Banks
Acute pancreatitis, an inammatory disorder of the pancreas, is the leading cause of admission to hospital for Lancet 2015; 386: 8596
gastrointestinal disorders in the USA and many other countries. Gallstones and alcohol misuse are long-established Published Online
risk factors, but several new causes have emerged that, together with new aspects of pathophysiology, improve January 21, 2015
http://dx.doi.org/10.1016/
understanding of the disorder. As incidence (and admission rates) of acute pancreatitis increase, so does the demand
S0140-6736(14)60649-8
for eective management. We review how to manage patients with acute pancreatitis, paying attention to diagnosis,
This online publication has
dierential diagnosis, complications, prognostic factors, treatment, and prevention of second attacks, and the possible been corrected. The corrected
transition from acute to chronic pancreatitis. version rst appeared at
thelancet.com on
November 19, 2015
Introduction In four large retrospective studies, type 2 diabetes
In this Seminar, we provide a comprehensive and increased the risk of acute pancreatitis by 186289 Department of General Internal
Medicine and
balanced account of the advances since the 2008 times.1215 Compared with non-diabetics, the risk was Gastroenterology, Clinical
Seminar in The Lancet on acute pancreatitis,3 highlight particularly high in younger patients with diabetes Centre of Lneburg, Lneburg,
areas of controversy or international dierences in (incidence rate ratio 526 in those younger than 45 years Germany (Prof P G Lankisch MD);
Pancreatic Research Group,
practice, and describe concepts underlying the disease. [95% CI 431642]; 244 in those 45 years and older
South Western Sydney Clinical
The annual incidence of acute pancreatitis ranges from [223266]),15 and the excess risk was reduced by School, Faculty of Medicine,
13 to 45 per 100 000 people (appendix).4 In patients antidiabetic drugs.14 The possibility of incretin-based University of New South Wales,
treated in hospital in the USA in 2009, acute pancreatitis therapies leading to acute pancreatitis is being debated.16,17 Sydney, NSW, Australia
(Prof M Apte PhD); Ingham
was the most frequent principal discharge diagnosis Whether failure of fusion of the dorsal and ventral
Institute for Applied Medical
in gastrointestinal disease and hepatology.5 The pancreatic buds during gestation has any clinical or Research, Liverpool Hospital,
number of discharges with acute pancreatitis as pathological results is unknown. In a group of patients Liverpool, NSW, Australia
principal diagnosis was 30% higher than in 2000. with acute and chronic pancreatitis, the prevalence of (M Apte); and Division of
Gastroenterology, Hepatology,
Acute pancreatitis was the second highest cause of pancreas divisum was similar in those with and without
and Endoscopy, Harvard
total hospital stays, the largest contributor to aggregate idiopathic (75%) and alcoholic (7%) pancreatitis, Medical School, and Brigham
costs, and the fth leading cause of in-hospital showing that pancreas divisum alone does not cause and Womens Hospital, Boston,
deaths, showing the importance of accurate data for the disease.18 However, associations between pancreas MA, USA (Prof P A Banks MD)
the disorder. divisum and mutations of cystic brosis transmembrane Correspondence to:
Prof Paul Georg Lankisch,
conductance regulator (CFTR) of 47%, serine protease
Department of General Internal
Causes inhibitor Kazal-type 1 of 16%, or protease, serine 1 of Medicine and Gastroenterology,
Gallstones and alcohol misuse are the main risk factors 16%, were noted, suggesting a cumulative eect. This Clinical Centre of Lneburg,
for acute pancreatitis (appendix). During 2030 years, conclusion is not straightforward, however, because Reiherstieg 23,
D-21337 Lneburg, Germany
however, the risk of biliary pancreatitis is unlikely to be associations do not necessarily mean causation.
paulgeorg.lankisch@t-online.de
more than 2% in patients with asymptomatic gallstones6 Patients with pancreas divisum and CFTR mutations
and that of alcoholic pancreatitis is unlikely to exceed should be referred for genetic counselling, and See Online for appendix
23% in heavy drinkers.7 Other factors, possibly genetic, endoscopic or surgical therapy should be withheld
therefore probably play a part. Drugs represent an unless randomised studies show benet.19
additional cause of acute pacreatitis8 (panel 1 and Pancreatitis is the most frequent complication after
appendix). endoscopic retrograde cholangiopancreatography (fre-
Smoking might increase the risk of acute pancreatitis.911 quency 35% in unselected patients).20 It is mild or
There is no association between smoking and biliary
pancreatitis, but the risk of non-gallstone-related acute
pancreatitis has been shown to more than double Search strategy and selection criteria
(relative risk 229, 95% CI 163322) in present We searched PubMed for the term acute pancreatitis,
smokers with 20 or more pack-years compared with together with aetiology, pathogenesis, prognostic
never-smokers. Notably, in heavy smokers with a parameters, complications, death, treatment, or
consumption of 400 or more grams of alcohol per month, prognosis. We included articles in English, French, German,
the risk increased by more than four times (412, and Spanish from Jan 1, 2009 to Dec 31, 2013, together with
198860). Smoking duration rather than intensity highly cited older publications that seemed necessary for full
increased the risk. It was benecial to stop smoking, understanding. Moreover, we included several sets of
but only after two decades was the risk similar to guidelines, two of which cover almost the whole range of
non-smokers. These ndings9 could show that smoking acute pancreatitisnamely, those from the American College
is an independent risk factor for acute pancreatitis, but of Gastroenterology1 and the International Association of
residual confounding factors and missing alcohol intake Pancreatology and American Pancreatic Association.2
data are limitations of the study.
www.thelancet.com Vol 386 July 4, 2015 85
Seminar
prospective studies are needed to ascertain the true
Panel 1: Drugs for which a denite or probable association with acute pancreatitis incidence of acute pancreatitis and potentially identify
has been reported (up to 2011) avoidable risk factors after double-balloon and single-
Denite balloon enteroscopy.
Acetaminophen, asparaginase, azathioprine, bortezomib, capecitabine, carbomazepine,
cimetidine, cisplatin, cytarabine, didanosine, enalapril, erythromycin, oestrogens, Pathogenesis
furosemide, hydrochlorothiazide, interferon alfa, itraconazole, lamivudine, Mechanisms of cellular injury
mercaptopurine, mesalazine, olsalazine, methyldopa, metronidazole, octreotide, Pancreatic duct obstruction, irrespective of the mechanism,
olanzapine, opiates, oxyphenbutazone, pentamidine, pentavalent antimony compounds, leads to upstream blockage of pancreatic secretion, which
penformin, simvastatin, steroids, sulfasalazine, co-trimoxazole in turn impedes exocytosis of zymogen granules
(containing digestive enzymes) from acinar cells.
Probable Consequently, the zymogen granules coalesce with
Atorvastatine, carboplatin, docetaxel, ceftriaxon, cyclopenthiazide, didanosine, intracellular lysosomes to form condensing or autophagic
doxycycline, enalapril, famotidine, ifosfamide, imatinib, liraglutide, maprotiline, vacuoles containing an admixture of digestive and
mesalazine, orlistat, oxaliplatine, rifampin, secnidazole, sitagliptine, sorafenib, tigecyclin, lysosomal enzymes. The lysosomal enzyme cathepsin B
vildagliptine, sulindac, tamoxifen, tetracycline, valproate can activate the conversion of trypsinogen to trypsin.
Modied with permission from reference 8. Findings from studies show lysosomal dysfunction in
pancreatitis and an imbalance between the trypsinogen-
activating isoform cathepsin B and the trypsin-degrading
Adjusted odds ratios (95% CI) Pooled incidence of PEP isoform cathepsin L.23 The resulting accumulation of active
(patients with vs those trypsin within the vacuoles can activate a cascade of
without risk factor) digestive enzymes leading to autodigestive injury (a concept
Patient-related risk factors rst proposed by Hans Chiari24). A block in the healthy
Denite risk factors apical exocytosis of zymogen granules can cause basolateral
Suspected sphincter of Oddi dysfunction 409 (337496) 103% vs 39% exocytosis in the acinar cell, releasing active zymogens into
Female sex 223 (175284) 40% vs 21% the interstitial space (rather than the acinar lumen), with
Previous pancreatitis 246 (193312) 67% vs 38% subsequent protease-induced injury to the cell mem-
Likely risk factors branes.25 Evidence supporting a role for premature
Younger age 109287 (range 109668) 61% vs 24% trypsinogen activation and autodigestion in acute
Non-dilated extrahepatic bile ducts Not reported 65% vs 67% pancreatitis comes from the discovery in patients with
Absence of CP 187 (100348) 40% vs 31% hereditary pancreatitis of a mutation in the trypsinogen
Normal serum bilirubin 189 (122293) 100% vs 42% gene, resulting in the formation of active trypsin that is
Procedure-related risk factors resistant to degradation.26 Genetically engineered mice with
Denite risk factors
an absence of the trypsinogen 7 gene are protected from
Precut sphincterotomy 271 (202363) 53% vs 31%
supramaximal caerulein-induced acinar injury, which
Pancreatic injection 22 (160301) 33% vs 17%
supports this theory.26
Acinar injury due to autodigestive processes stimulates
Likely risk factors
an inammatory response (inltration of neutrophils and
High number of cannulation attempts 240341 (range 107567) 37% vs 23%
macrophages, and release of cytokines tumour necrosis
Pancreatic sphincterotomy 307 (164575) 26% vs 23%
factor and interleukins 1, 6, and 8) within the pancreatic
Biliary balloon sphincter dilation 451 (1511346) 93% vs 19%
parenchyma. However, parenchymal inammation has
Failure to clear bile duct stones 335 (133910) 17% vs 16%
also been shown in trypsinogen-null mice after caerulein
PEP=postendoscopic retrograde cholangiopancreatography. CP=chronic pancreatitis. hyperstimulation,27 suggesting that inammatory inl-
tration can occur independent of trypsinogen activation.
Table 1: Independent risk factors for PEP20
Whatever the stimulus for inammation, in a few cases
the reaction is severe, with multiorgan failure and sepsis;
moderate in about 90% of cases. Independent patient- sepsis is particularly thought to result from an increased
related and procedure-related risk factors for propensity for bacterial translocation from the gut lumen
postendoscopic retrograde cholangiopancreatography to the circulation.28
pancreatitis act synergistically (table 1). The toxic eects of bile acid itself on acinar cells have
Single-balloon or double-balloon enteroscopy can result attracted attention as a possible pathogenetic factor in
in hyperamylasaemia and acute pancreatitis, probably biliary pancreatitis. Bile acids can be taken up by acinar
because of repeated stretching of the small-bowel or cells via bile acid transporters located at apical and
mesenteric ligaments. The rates of hyperamylasaemia are basolateral plasma membranes29 or by a G-protein-coupled
reported to be 17% for double-balloon enteroscopy and receptor for bile acids (Gpbar1).30 Once within the cell,
16% for single-balloon enteroscopy, but the rate of acute bile acids increase intra-acinar calcium concentrations
pancreatitis is much lower, at no more than 1%.21,22 Large via inhibition of sarcoendoplasmic Ca+-ATPase and
86 www.thelancet.com Vol 386 July 4, 2015
Seminar
activate signalling pathways, including MAPK and PI3K,
and transcription factors such as NF-B, thereby
inducing synthesis of proinammatory mediators.31
However, whether these processes are clinically impor- Necrosis
tant remains unclear since clinical evidence for
Cytokines
biliopancreatic reux is scarce.
ROS
Acinar cell
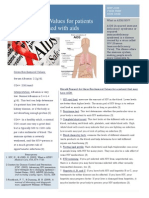
Alcoholic pancreatitis
Alcohol is known to exert direct toxic eects on the Stellate cell
ZG
pancreas, but additional triggers or cofactors seem to be activation L
necessary to initiate overt pancreatitis. Early studies Stellate cell GP2
focused on the eects of alcohol on the sphincter of Oddi
as a possible mechanism of duct obstruction leading to Ca2+
pancreatitis (similar to that for biliary pancreatitis).
However, the results were controversial, with both Mitochondrial
decreased and increased sphincter of Oddi tone reported.32 Oxidant stress depolarisation
There is more consistent evidence that the eects of
alcohol on small pancreatic ducts and the acinar cells Mitochondrion
themselves play a part in alcohol-induced pancreatic mRNA
injury.32 Alcohol increases the propensity for precipitation CE and FAEE RER
of pancreatic secretions and the formation of protein Ac
plugs within pancreatic ducts owing to changes of
lithostathine and glycoprotein 2, two non-digestive en-
Ethanol
zyme components of pancreatic juice with self-aggregation
properties; and to increased viscosity of pancreatic
Figure: Eects of alcohol on the pancreatic acinar and stellate cell, on the basis of experimental in-vitro and
secretions because of CFTR dysfunction.32,33 The protein in-vivo evidence
plugs enlarge and form calculi, causing ulceration of Pancreatic acinar cells metabolise alcohol via both oxidative and non-oxidative pathways, and exhibit changes that
adjacent ductal epithelium, scarring, further obstruction, predispose the cells to autodigestive injury, necroinammation, and cell death. These changes include:
destabilisation of lysosomes and zymogen granules (mediated by oxidant stress [ROS, CE, FAEE, and decreased
and, eventually, acinar atrophy and brosis.33
GP2, a major structural component of zymogen membranes); increased digestive and lysosomal enzyme content
Experimental studies have shown that alcohol increases (because of increased synthesis [increased mRNA] and impaired secretion); increased activation of transcription
digestive and lysosomal enzyme content within acinar factors (NF-B and AP-1) that regulate cytokine expression; and a sustained increase in cytoplasmic Ca+ and
cells and destabilises the organelles that contain these mitochondrial Ca+ overload, leading to mitochondrial depolarisation. Pancreatic stellate cells have the capacity to
oxidise alcohol to acetaldehyde, which is associated with the generation of reactive oxygen species, leading to
enzymes,34 thereby increasing the potential for contact oxidant stress. Pancreatic stellate cells are activated, on exposure to alcohol, to a myobroblast-like phenotype,
between digestive and lysosomal enzymes, and facilitating stimulating synthesis of proinammatory mediators and cytokines by the cells. This sensitises the pancreas such
premature intracellular activation of digestive enzymes. that in the presence of an appropriate trigger or cofactor, overt injury is initiated. The eects of ethanol on acinar
These eects of alcohol on acinar cells are probably a cells are represented by red arrows and on stellate cells by green arrows. Ca+=calcium. Ac=acetaldehyde.
CE=cholesteryl esters. FAEE=fatty acid ethyl esters. GP2=glycoprotein 2. L=lysosomes. RER=rough endoplasmic
result of the metabolism of alcohol within the cells, reticulum. ROS=reactive oxygen species. ZG=zymogen granules. Adapted with permission from reference 32.
leading to the generation of toxic metabolites
(acetaldehyde, fatty acid ethyl esters, and reactive oxygen alcoholic acute pancreatitis is particularly contro-
species) and changes in the intracellular redox state versial35,36 because although animal studies have shown
(appendix, gure). detrimental eects of cigarette smoke extract, nicotine,
Alcohol exerts toxic eects on pancreatic stellate cells and nicotine-derived nitrosamine ketone on duct or
(resident cells of the pancreas that regulate healthy acinar cells,3739 the clinical relevance of these ndings is
extracellular matrix turnover).32 PSCs are activated by mitigated by the very close association between heavy
alcohol, its metabolites, and oxidative stress to convert smoking and drinking, making it dicult to ascribe the
into a myobroblast-like phenotype that synthesises initiation of acute pancreatitis in human beings to
cytokines, which can contribute to the inammatory smoking alone. Nevertheless, there is general con-
process during acute pancreatitis (gure). sensus that smoking accelerates the progression of
Despite the known detrimental eects of alcohol and alcoholic pancreatitis.40 Bacterial endotoxinaemia is
its metabolites on the pancreas, only a few drinkers another possible trigger factor, as shown by exper-
develop overt disease, prompting a search for the imental evidence that an endotoxin challenge in
additional insult needed for precipitating pancreatitis. alcohol-fed rats leads to acute pancreatitis, whereas
Unfortunately, none of the candidate trigger factors alcohol feeding alone causes no damage.41 Since alcohol
investigated so far (diet, amount and type of alcohol is known to increase gut permeability, an inability to
consumed, pattern of alcohol consumption, presence of detoxify circulating endotoxin could make some
hyperlipidaemia, smoking, and inherited factors) have drinkers susceptible to overt disease.
been shown to have a clear role. The role of smoking in Genetic factors related to digestive enzymes, trypsin
www.thelancet.com Vol 386 July 4, 2015 87
Seminar
inhibitors, cytokines, CFTR, MHC antigens, alcohol- classication recommends that the modied Marshall
metabolising enzymes, oxidant stress-related proteins, scoring system should be used to characterise the
and detoxifying enzymes have not shown an association severity of failure of these three systems. Systemic
with alcoholic pancreatitis. Investigators of a genome-wide complications are dened as exacerbations of
association study reported an association between pre-existing comorbidities, including congestive heart
overexpression of claudin 2 (a tight-junction protein) and failure, chronic liver disease, and chronic lung disease.
increased risk of alcoholic pancreatitis, with the protein Local complications include interstitial pancreatitis
overexpressed on the basolateral membranes of acinar (peripancreatic uid collections and pancreatic pseudo-
cells in these patients.42 However, the functional cysts) and necrotising pancreatitis (acute necrotic
signicance of this nding remains unclear. collections and walled-o necrosis; panel 2). Patients
A nal aspect of pathogenesis is the multitude of who have moderately severe acute pancreatitis might
signalling pathways and molecules that are perturbed need a longer stay in hospital and have a higher
within the acinar cell upon exposure to injurious agents, mortality than patients with mild acute pancreatitis.
but accumulating evidence points to aberrant intracellular Severe acute pancreatitis is characterised by the
calcium signalling as the nal common mechanism for presence of persistent single-organ or multiorgan failure
acinar injury (appendix).43,44 (dened by organ failure that is present for 48 h). Most
patients who have persistent organ failure have
Classication pancreatic necrosis and a mortality of at least 30%.
The Atlanta classication45 is the standard classication of An alternative stratication of acute pancreatitis severity
the severity of acute pancreatitis. The recently published has been proposed, which includes four categories rather
revised classication46 provides denitions of the clinical than three (table 2).47 These are mild (absence of necrosis
and radiologic severity of acute pancreatitis. Clinical severity or organ failure), moderately severe (sterile necrosis and/
of acute pancreatitis is stratied into three categories: mild, or transient organ failure), severe (infected necroses or
moderately severe, and severe (table 2). persistent organ failure), and critical (infected necroses
Patients with mild acute pancreatitis (no organ failure and persistent organ failure). Studies will be needed to
or systemic or local complications) usually do not need ascertain whether it is more clinically relevant to stratify
pancreatic imaging and are frequently discharged within patients into these three or four categories of severity.
37 days of onset of illness. For radiological severity of acute pancreatitis, the
Moderately severe acute pancreatitis is characterised revised classication provides detailed denitions of the
by one or more of transient organ failure (dened as imaging features of the disease. Acute peripancreatic
organ failure lasting <48 h), systemic complications, or uid collections occur within the rst several days of
local complications. Organ failure includes respiratory, interstitial pancreatitis. They are homogeneous in
cardiovascular, and renal failure using the same criteria appearance, usually remain sterile, and most often resolve
as in the Atlanta Symposium of 1992.45 The revised spontaneously. An acute peripancreatic uid collection
that does not resolve can develop into a pseudocyst, which
contains a well dened inammatory wall. There is very
Atlanta classication 199245 Revised Atlanta Determinant-based little, if any, solid material within the uid of a pseudocyst.
classication 201246 classication 201247
Of particular importance is the radiological denition of
Mild No organ failure and no local No organ failure and no No (peri)pancreatic acute necrotic collections and walled-o necrosis.
complications local or systemic necrosis and organ failure
Previously, the site of acute necrotic collections in necro-
complications
tising pancreatitis was thought to include the pancreatic
Moderately severe .. Transient organ failure Sterile (peri)pancreatic
(<48 h) and/or local or necrosis and/or transient parenchyma and peripancreatic tissue or, on rare occas-
systemic complications organ failure (<48 h) ions, only the pancreatic parenchyma. It is now recognised
without persistent organ that acute necrotic collection can include only the
failure (>48 h)
peripancreatic tissue. Patients with peripancreatic necrosis
Severe Local complications and/or Persistent organ failure Infected (peri)pancreatic
organ failure: PaO2 60% or (>48 h):* single organ necroses or persistent
have an increased morbidity and mortality compared with
creatinine 1526 mol/L or failure or multiple organ organ failure (>48 h) interstitial pancreatitis. Acute necrotic collections in necro-
shock (systolic blood pressure failure tising pancreatitis can be sterile or infected. The natural
60 mm Hg) or
history of acute necrotic collections is variable. They can
gastrointestinal bleeding
(>500 mL/24 h) become smaller and, on rare occasions, wholly disappear.
Critical .. .. Infected (peri)pancreatic Most often, acute necrotic collections develop a well dened
necroses and persistent inammatory wall surrounding varying amounts of uid
organ failure and necrotic debristermed walled-o necrosiswhich
Neither Atlanta classications have a fourth critical group; this group is solely in the determinant-based can be either sterile or infected.
classication. *Persistent organ failure is now dened by a modied Marshall score (appendix).48 This revised classication needs to be tested to assess
its clinical usefulness, and is likely to undergo further
Table 2: Denition of severity in acute pancreatitis.
revisions in the future. The appendix lists clinical
88 www.thelancet.com Vol 386 July 4, 2015
Seminar
Panel 2: Revised denitions of morphological features of acute pancreatitis
Interstitial oedematous pancreatitis CECT criteria
Acute inammation of the pancreatic parenchyma and Well circumscribed; usually round or oval.
peripancreatic tissues, but without recognisable tissue Homogeneous uid density.
necrosis. No non-liquid component.
CECT criteria Well dened wall that is wholly encapsulated.
Pancreatic parenchyma enhancement by intravenous Maturation usually needs >4 weeks after onset of acute
contrast agent. pancreatitis; occurs after interstitial oedematous
No peripancreatic necrosis. pancreatitis.
Necrotising pancreatitis Acute necrotic collection
Inammation associated with pancreatic parenchymal necrosis A collection containing variable amounts of both uid and necrosis
and/or peripancreatic necrosis. associated with necrotising pancreatitis; the necrosis can include
CECT criteria the pancreatic parenchyma and/or the peripancreatic tissue.
Lack of pancreatic parenchymal enhancement by CECT criteria
intravenous contrast agent. Occurs only in the setting of acute necrotising
Presence of ndings of peripancreatic necrosis. pancreatitis.
Heterogeneous and non-liquid density of varying
Acute pancreatitis uid collection
degrees in dierent locations (some seem homogeneous
Peripancreatic uid associated with interstitial oedematous
early in their course).
pancreatitis with no associated peripancreatic necrosis. Applies
No denable wall encapsulating the collection
only to areas of peripancreatic uid seen within the rst
Intrapancreatic and/or extrapancreatic.
4 weeks after onset of interstitial oedematous pancreatitis and
without the features of a pseudocyst. Walled-o necrosis
CECT criteria A mature, encapsulated collection of pancreatic and/or
Occurs in the setting of interstitial oedematous peripancreatic necrosis that has developed a well dened
pancreatitis. inammatory wall. Usually occurs >4 weeks after onset of
Homogeneous collection with uid density. necrotising pancreatitis.
Conned by normal peripancreatic fascial planes. CECT criteria
No denable wall encapsulating the collection. Heterogeneous with liquid and non-liquid density, with
Adjacent to pancreas (no intrapancreatic extension). varying locations (some can seem homogeneous)
Well-dened wall that is wholly encapsulated.
Pancreatic pseudocyst
Intrapancreatic and/or extrapancreatic.
An encapsulated collection of uid with a well dened
Maturation usually needs 4 weeks after onset of acute
inammation wall, usually outside the pancreas, with little or
necrotising pancreatitis.
no necrosis. Usually occurs more than 4 weeks after onset of
interstitial oedematous pancreatitis. CECT=contrast-enhanced CT. Reproduced with permission from reference 46.
presentation and physical examination, and the essential concentrations on admission are not associated with
abdominal and systemic complications of acute disease severity.49 The disease can be serious, even fatal,
pancreatitis. although the enzymes are only slightly increased (<three-
times normal).
Diagnosis
Main diagnostic procedures Laboratory tests
Clinicians are interested in conrmation of the diagnosis In addition to serum amylase and lipase, the following
and exclusion of dierential diagnoses (appendix). In variables should be established on admission: complete
accordance with the revised Atlanta classication, acute blood count without dierential; concentrations of
pancreatitis can be diagnosed if at least two of the following electrolytes, blood urea nitrogen (BUN), creatinine,
three criteria are fullled: abdominal pain (acute onset of serum glutamic pyruvic transaminase, serum glutamic
persistent and severe epigastric pain, often radiating to the oxalic transaminase, alkaline phosphatase, and blood
back); serum lipase (or amylase) activity at least three times sugar; coagulation status; and total albumin. Arterial
the upper limit of normal; or characteristic ndings of blood gas analysis is generally indicated whenever
acute pancreatitis on contrast-enhanced CT or, less often, oxygen saturation is less than 95% or the patient is
MRI or transabdominal ultrasonography.46 Diagnostic tachypnoeic. The frequency of repeat determinations
imaging is essential in patients with a slight enzyme depends on the clinical course.
elevation (appendix). Importantly, pancreatic enzyme
www.thelancet.com Vol 386 July 4, 2015 89
Seminar
ECG and chest radiograph One such approach is the harmless acute pancreatitis
50% or fewer cases of ST segment elevations and score (HAPS), which enables identication of mild cases
negativities are registered, mainly in the posterior wall, of acute pancreatitis (which is most of them) within
without myocardial infarction. Chest radiographs in 30 min of inpatient admission, even by non-specialists.
two planes can show pleural eusions and pulmonary Two prospective studies,60 one monocentric and the other
inltrates, which are signs of severe disease. Abdominal multicentric, showed that mild acute pancreatitis can be
panoramic radiographs (upright or left lateral position) predicted with 98% accuracy in patients with no rebound
can be used for diagnosis too. Ileus is shown by a sentinel tenderness or guarding and normal haematocrit and
loop (isolated bowel loop in left-upper or middle abdomen) serum creatinine concentrations. Studies from Sweden61
or colon cuto sign (absence of air in left colonic exure and India62 support the accuracy of HAPS. This score
or descending colon). Pancreatic calcications represent thus identies most patients who have neither developed,
proof of chronic pancreatitisie, that the patient is having or will develop, necrotising pancreatitis or organ failure,
an episode of acute superimposed on chronic pancreatitis, and will therefore not need intensive care. HAPS can be
rather than a rst episode of acute pancreatitis. used in the community care setting, in which the treating
physician can triage the patients who need early transfer
CT to more specialised centres for more aggressive
Unenhanced CT scoring systems assess the extent of management and meticulous monitoring.62 The score
pancreatic and peripancreatic inammatory changes might even be able to establish whether the patient could
(Balthazar score50 or pancreatic size index51), or both be cared for adequately and more economically as an
peripancreatic inammatory changes and extrapancreatic outpatient.
complications (mesenteric oedema and peritoneal uid
score,52 extrapancreatic score,53 or extrapancreatic Therapy
inammation on CT score).54 The patients management begins on the emergency
Two CT scoring systems need intravenous contrast ward, where acute pancreatitis has to be conrmed,
agents to establish the presence and extent of pancreatic the risk stratied, and basic treatment initiated. This
parenchymal necrosis. The CT severity index55 combines treatment includes early uid resuscitation, analgesia,
quantication of extrapancreatic inammation with extent and nutritional support (appendix). Patients undergoing
of pancreatic necrosis, whereas the modied CT severity volume resuscitation should have the head of the bed
index56 assigns points for extrapancreatic (eg, vascular, raised, undergo continuous pulse oximetry, and receive
gastrointestinal, or extrapancreatic parenchymal) compli- supplemental oxygen. Supplemental oxygen has been
cations and presence of pleural eusions or ascites. shown to more than half mortality in patients older than
Contrast-enhanced CT is the gold standard for 60 years.63
diagnostic imaging to help to establish disease severity In experimental pancreatitis in the rat, pancreatic
(the appendix contains axial contrast-enhanced CT microvascular perfusion is reduced, which is aggravated
scans of the pancreas of a patient with acute pancreatitis by arterial hypotension.64 The situation in human beings,
on admission and 1, 10, and 20 days later). However, the however, remains unclear. Neither comparisons of
predictive accuracy of CT scoring systems for severity aggressive versus non-aggressive resuscitation protocols
of acute pancreatitis is similar to clinical scoring (4 L vs 35 L within the rst 24 h) nor goal-directed uid
systems. A CT scan on admission solely for severity therapy (goals have included BUN concentration, central
assessment in acute pancreatitis is therefore not venous pressure, haematocrit concentration, heart rate,
recommended.57 An early CT scanie, done within the blood pressure, and urine output) have yielded clear
rst 4 full days after symptom onset (days 04)does results.65 The investigators of one retrospective study
not show an alternative diagnosis, help with the showed that early uid resuscitation was associated with
distinction of interstitial versus necrotising pancreatitis, reduced incidence of systemic inammatory response
or provide evidence of an important complication.58 An syndrome and organ failure at 72 h,66 but too little uid is
early CT scan should therefore be obtained only when just as deleterious as too much. In one study, rapid
there is clinical doubt about the diagnosis of acute haemodilution increased both the incidence of sepsis
pancreatitis, and other life-threatening disorders have within 28 days and in-hospital mortality.67 In another, the
to be excluded. administration of a small amount of uid was not
associated with a poor outcome, but the need for a large
Prognostic variables amount of uid was.68
Existing scoring systems (appendix) seem to have With regard to what should be infused, the recommend-
reached their maximum eectiveness in the prediction ations of the American College of Gastroenterology
of persistent organ failure in acute pancreatitis. (ACG) and International Association of Pancreatology
Sophisticated combinations of predictive rules are more (IAP)/American Pancreatic Association (APA) guidelines
accurate, but cumbersome, and therefore of restricted are very similar: ACG suggests that lactated Ringers
clinical use, and new approaches are needed.59 solution might be preferred to isotonic crystalloid
90 www.thelancet.com Vol 386 July 4, 2015
Seminar
replacement uid,1 whereas IAP/APA merely state2 that supplementary nutrition. Enteral nutrition was associated
Ringers lactate should not be given to the few patients with a lower risk of complications than parenteral
with hypercalcaemia for initial uid resuscitation. The nutrition, but not with a signicant change in mortality.
two sets of guidelines dier with regard to rate of However, timing is crucial. The investigators of a
infusion, with ACG suggesting a rate of 250500 mL/h systematic review of 11 RCTs showed that when started
and IAP/APA suggesting 510 mL/kg per h. If the ACG within 48 h of admission, but not later, enteral nutrition,
recommendation is assumed to be for a patient weighing compared with parenteral nutrition, signicantly reduces
70 kg, following the IAP/APA guideline would lead to a the risk of multiorgan failure, pancreatic infectious
much higher rate of infusion, of 50700 mL/h. Only ACG complications, and mortality.77 Many studies have
makes a rm recommendation as to when infusion proposed that enteral nutrition should be given via a
should begin, stating that early aggressive intravenous nasoduodenal rather than nasojejunal tube, but a rm
hydration is most benecial in the rst 1224 h and could recommendation cannot yet be given.7881 An initial
have little benet beyond this time.1 attempt at nasoduodenal intubation seems advisable, but
These recommendations are essentially based on a the pancreatic head inammation in severe acute
prospective multicentre randomised study69 in which pancreatitis can cause duodenal stenosis, necessitating
resuscitation with lactated Ringers solution reduced by endoscopic placement. Nausea and vomiting because of
84% during the rst 24 h compared with normal saline. persisting gastroparesis, ileus, or postprandial pain
Infusion started with a bolus of 20 mL/kg bodyweight suggests parenteral nutrition via a central venous catheter.
followed by 3 mL/kg for 812 h. Crucial, however, is Glutamine supplementation has been discussed for
adjustment of the infusion rate depending on the results of patients with critical acute pancreatitis leading to
measurements of intervals of no more than 6 h for at least catabolism. Findings from a meta-analysis of 12 RCTs82
2448 h. One decisive variable is BUN because investigators showed that glutamine supplementation signicantly
have shown that increased BUN concentration at admission reduced the risk of mortality and total infectious
and during the rst 24 h are independent risk factors for complications in parenterallybut not enterallyfed
mortality in acute pancreatitis.70,71 The recommendation has patients, but did not shorten the hospital stay. The
been made to adjust uid resuscitation during the rst 24 h absence of a positive eect of enteral glutamine
on the basis of whether BUN concentration increases supplementation was attributed to the fact that
or decreases.72 glutamine is largely metabolised in the gut and liver so
Pain treatment has absolute priority on admission. that the plasma glutamine concentration is lower after
Unfortunately, ndings from a systematic review enteral than after intravenous administration. An
showed that the randomised controlled trials (RCTs) additional point to note is that treatment with
comparing dierent analgesics were of low quality and antioxidants is ineective.8385
did not clearly favour any particular analgesic for pain A Cochrane review86 showed no evidence that routine
relief.73 Until a conclusive study is reported, the early endoscopic retrograde cholangiopancreatography
prevailing guidelines for acute pain management in the signicantly aects mortality and local or systemic
perioperative setting should be followed.74 complications in patients with acute gallstone pancreatitis,
Patients in high-volume centres (118 admissions per irrespective of predicted severity. The results, however,
year) have a 25% lower relative risk of death than do support present recommendations86 that early endoscopic
those in low-volume centres.75 Patients who do not retrograde cholangiopancreatography should be considered
respond to early resuscitation or display persisting organ in patients with coexisting cholangitis or biliary obstruction.
failure or widespread local complications should
therefore be transferred to a pancreatitis centre (if Management of local complications
available) with multidisciplinary expertise, including Necrosis
therapeutic endoscopy, interventional radiology, and Prophylactic antibiotics are not indicated.8790 Surgical
surgery. Patients with persistent systemic inammatory resection of pancreatic necroses can be achieved by
response syndrome, increased concentrations of BUN or open, laparoscopic, or staged necrosectomy (open-staged
creatinine, increased haematocrit, or underlying cardiac or closed-continuous lavage). These methods do not
or pulmonary illness, should be admitted for compete with, but rather complement, other techniques.
monitoringeither intensive or intermediate care, No guidelines exist, but there is consensus that surgical
depending on availability. All other patients, especially intervention should be doneif at allat a late stage, at
those in whom HAPS60 predicts harmless acute least 2 weeks after the onset of pancreatitis.91
pancreatitis, can be treated on a general ward. More conservative interventions than surgery now
In mild acute pancreatitis, oral feedings can be started predominate92,93 as a result of two pioneering advances.
if there is no nausea and vomiting, and abdominal pain Antibiotic treatment alone can heal infected necrosis.94
has resolved.1 Findings from a systematic review of This is now the rst step when such lesions are shown.
15 RCTs76 showed that either enteral or parenteral Antibiotic treatment is possible in almost two-thirds of
nutrition is associated with a lower risk of death than no patients with necrotising pancreatitis, with a mortality of
www.thelancet.com Vol 386 July 4, 2015 91
Seminar
7%.95 Seifert and colleagues96 successfully introduced mortality.108,109 If sepsis is suspected, it is reasonable to start
debridement of infected necrosis after fenestration of the antibiotics while waiting for blood culture results. If
gastric wall. This form of intervention has become widely culture results are negative, antibiotics should be
used and other routes of access have been developed, but discontinued to reduce the risk of fungaemia110 or
it should be restricted to specialist centres. Long-term Clostridium dicile infection.72
success can then be achieved in two-thirds of patients.97
Endoscopic transgastric necrosectomy compares favour- Aftercare
ably with surgery.98 Clinical trials are needed to validate Refeeding
the various options for intervention. Basic treatment of acute pancreatitis should be
Van Santvoort and colleagues99 compared step-up continued until the patient shows distinct clinical
management of infected necrosis (placement of improvement (freedom from pain and normal body
percutaneous catheters in addition to treatment with temperature and abdominal ndings). No binding
antibiotics, if necessary followed by minimally invasive recommendation for severe acute pancreatitis exists; the
necrosectomy) with open necrosectomy. This step-up decision is taken on an individual basis. In mild acute
approach reduced new-onset multiorgan failure by 29%. pancreatitis, oral feeding should be resumed as soon as
However, the study was underpowered to detect a possible according to the present European Society for
dierence in mortality. Parenteral and Enteral Nutrition guidelines.111 When and
In patients with walled-o necrosis, physicians should how this feeding should be resumed remains undened.
intervene only in the event of symptoms attributable to The beginning of refeeding certainly does not depend on
the collection (persistent abdominal pain, anorexia, the normalisation of lipase.112 The decision should
nausea, or vomiting from mechanical obstruction or perhaps be left to the patientsie, they can eat when
secondary infection).72 In this case, direct endoscopic they are hungry.112,113 Positive experience with refeeding at
necrosectomy is possible in skilled hands.100 the patients request has been reported with widely
varying diets: unspecied,114 soft diet,115 and full diet
Pseudocyst with116 or without117 fat restriction. Unfortunately,
Prognostic factors for the development of pseudocysts are however, oral refeeding can lead to pain relapse and
alcohol misuse and initially severe disease. Spontaneous therefore to a longer hospital stay (appendix).
resolution occurs in a third of patients with a pseudocyst.
Prognostic factors for this resolution are no or mild Imaging procedures
symptoms, and a pseudocyst diameter of no more than Patients with acute pancreatitis of unknown origin
4 cm.101 Symptomatic pseudocysts can be successfully should undergo endosonography to exclude stones or
decompressed by endoscopic cystogastrostomy with sludge in the gallbladder or bile ducts. Endosonography
endoscopic ultrasound guidance.102 or magnetic resonance cholangiopancreatography can be
indicated to exclude a tumour. Tumour-related acute
Ductal disruption pancreatitis can seem to heal before aring up again.118
Ductal disruption can result in unilateral pleural eusion,
pancreatic ascites, or enlarging uid collection. If the Transient exocrine and endocrine pancreatic insuciency
disruption is focal, placement of a bridging stent via Both exocrine and endocrine transient pancreatic
endoscopic retrograde cholangiopancreatography usually insuciency can occur during healing.119121
promotes duct healing.103 When ductal disruption occurs Pancreatic function should therefore be monitored,
in an area of widespread necrosis, optimum management which is generally normal again 3 months after
needs a multidisciplinary team of therapeutic endoscopists, abatement of acute pancreatitis. Pancreatic enzyme sub-
interventional radiologists, and surgeons.104 stitution is not usually necessary, but can be temporarily
necessary after a severe attack.
Peripancreatic vascular complications Endocrine pancreatic function should be checked after
Splenic vein thrombosis has been reported in up to 20% about 3 months (by fasting and postprandial blood sugar
of patients with acute pancreatitis undergoing imaging.105 concentrations, possibly by HbA1C measurement). Severe
The risk of bleeding from gastric varices is less than 5%, acute pancreatitis is often followed by diabetes mellitus.122
and splenectomy is not recommended. Pseudoaneurysms
are rare, but cause serious complications in 410% of Transition to chronic pancreatitis
cases.106 Mesenteric angiography with transcatheter In a German study,123 over a period of almost 8 years,
arterial embolisation is the rst-line treatment.107 only alcoholics developed chronic pancreatitis,
independently of both severity of rst acute pancreatitis
Management of extrapancreatic complications and discontinuation of alcohol and nicotine. The
Extrapancreatic infections, such as bloodstream infections, cumulative risk of the development of chronic
pneumonia, and urinary tract infections, occur early in up pancreatitis was 13% within 10 years and 16% within
to 24% of patients with acute pancreatitis, and can double 20 years. The risk of chronic pancreatitis in those who
92 www.thelancet.com Vol 386 July 4, 2015
Seminar
survived the second episode of acute pancreatitis was Conclusions
38% within 2 years. Nicotine misuse increased the risk From the pathophysiological viewpoint, the consensus
substantially. Similar investigations from Denmark124 has been that exposure of acinar cells to injurious agents
and the USA125 showed a transition to chronic from acute (alcohol or bile salts) perturbs a multitude of acinar
pancreatitis in 241% of patients after 35 years and functions (exocytosis, enzyme activation, lysosomal
323% after 34 years, respectively. In both studies, function, cytokine production, mitochondrial function,
transition also occurred occasionally in patients with and autophagy); however, ndings from studies suggest
non-alcohol-induced pancreatitis. that the nal common mechanism that mediates acinar
Ductal scarring can be seen on endoscopic retrograde cell death (irrespective of the cause of acute pancreatitis)
cholangiopancreatography, even after healing, but should, might be aberrant intracellular calcium signalling.44
under no circumstances, lead to diagnosis of chronic Novel evidence is accumulating to show that the
pancreatitis and substitution of pancreatic enzymes.126 pathogenesis of acute pancreatitis might not be limited
to acinar cell perturbation alone, but that pancreatic
Prevention stellate cells might also have a key early role, possibly via
One study127 showed that interventions by medical secretion of inammatory mediators upon activation, by
personnel (structured talks with patients by nurses factors such as alcohol and its metabolites.32,137
trained to inform patients how and why they should stay With regard to the clinical management of acute
abstinent) at 6-month intervals signicantly lowered the pancreatitis, the Atlanta classication has been revised46
recurrence rate of alcohol-induced pancreatitis within and will have to stand the test of clinical application. The
2 years. In patients with mild biliary acute pancreatitis, potential for new prognostic variables to assess severity
cholecystectomy should be done before discharge. In of pancreatitis seems to be exhausted. Great promise is
patients with necrotising biliary acute pancreatitis, shown by the novel HAPS, which, by contrast with the
cholecystectomy should be postponed to prevent existing variables, identies patients whose pancreatitis
infection until active inammation subsides and uid is only mild and who therefore do not need intensive
collections resolve or stabilise.1 In patients who cannot treatment. The past few years have seen particular interest
undergo surgery, the recurrence rate can be greatly in criteria for patient transfer, methods of uid
lowered by endoscopic sphincterotomy, with the aim of replacement and nutrition, and treatment of infected and
achieving spontaneous passage of any stones still present sterile necrosis, with implications for clinical practice.
in the gallbladder.128 Finally, attention has focused on the prevention of
Prophylactic stent placement and precut sphincterotomy repeated episodes of pancreatitis by alcohol weaning after
is recommended to prevent postendoscopic retrograde alcohol-induced acute pancreatitis and cholecystectomy
cholangiopancreatography pancreatitis.20 Findings from before discharge in patients with mild biliary acute
two meta-analyses129,130 show that prophylactic pancreatic pancreatitis, together with prevention of postendoscopic
stent placement reduces the risk of postendoscopic retrograde cholangiopancreatography pancreatitis by
retrograde cholangiopancreatography pancreatitis. Indo- means of rectal nonsteroidal anti-inammatory drugs or
methacin inhibits prostaglandin production in vivo, and is pancreatic stents.
a powerful inhibitor of serum phospholipase A2 activity in Contributors
acute pancreatitis. More than three decades ago, we All authors took part in the literature research, gure creation, data
showed that indomethacin given before or shortly after an analysis and interpretation, and manuscript writing. PGL coordinated
the project.
acute pancreatitis attack was triggered markedly reduced
mortality in rats.131 Later, the application of indomethacin Declaration of interests
We declare no competing interests.
suppositories reduced the frequency and intensity of pain
attacks in patients with acute pancreatitis.132 This Acknowledgments
MA is supported by project grants from the National Health and Medical
favourable eect of indomethacin was then forgotten, Research Council, and the Cancer Council of New South Wales, Australia.
until the recommendation of routine rectal administration
References
of 100 mg diclofenac or indomethacin immediately before 1 Tenner S, Baillie J, DeWitt J, Vege SS, and the American College of
or after endoscopic retrograde cholangiopancreatography20 Gastroenterology. American College of Gastroenterology guideline:
on the basis of ndings from three meta-analyses.133135 By management of acute pancreatitis. Am J Gastroenterol 2013;
108: 140015; 1416.
contrast, routine prophylactic use of nitroglycerin, 2 Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA
cephtazidime, somatostatin, gabexate, ulinastatin, gluco- evidence-based guidelines for the management of acute
corticoids, antioxidants, heparin, interleukin 10, pancreatitis. Pancreatology 2013; 13 (suppl 2): e115.
3 Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet 2008;
pentoxifylline, semapimod, and the recombinant platelet- 371: 14352.
activating factor acetylhydrolase is not recommended.20 4 Yadav D, Lowenfels AB. The epidemiology of pancreatitis and
The results of a network meta-analysis show that rectal pancreatic cancer. Gastroenterology 2013; 144: 125261.
non-steroidal anti-inammatory drugs are better than 5 Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal
disease in the United States: 2012 update. Gastroenterology 2012;
pancreatic duct stents for the prevention of postendoscopic 143: 117987, e13.
retrograde cholangiopancreatography pancreatitis.136
www.thelancet.com Vol 386 July 4, 2015 93
Seminar
6 Lowenfels AB, Lankisch PG, Maisonneuve P. What is the risk of 30 Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute
biliary pancreatitis in patients with gallstones? Gastroenterology pancreatitis in mice is mediated by the G-protein-coupled cell
2000; 119: 87980. surface bile acid receptor Gpbar1. Gastroenterology 2010; 138: 71525.
7 Lankisch PG, Lowenfels AB, Maisonneuve P. What is the risk of 31 Perides G, van Acker GJ, Laukkarinen JM, Steer ML. Experimental
alcoholic pancreatitis in heavy drinkers? Pancreas 2002; 25: 41112. acute biliary pancreatitis induced by retrograde infusion of bile
8 Nitsche C, Maertin S, Scheiber J, Ritter CA, Lerch MM, acids into the mouse pancreatic duct. Nat Protoc 2010; 5: 33541.
Mayerle J. Drug-induced pancreatitis. Curr Gastroenterol Rep 32 Apte MV, Pirola RC, Wilson JS. Mechanisms of alcoholic
2012; 14: 13138. pancreatitis. J Gastroenterol Hepatol 2010; 25: 181626.
9 Sadr-Azodi O, Andrn-Sandberg , Orsini N, Wolk A. Cigarette 33 Sarles H. Chronic calcifying pancreatitischronic alcoholic
smoking, smoking cessation and acute pancreatitis: a prospective pancreatitis. Gastroenterology 1974; 66: 60416.
population-based study. Gut 2012; 61: 26267. 34 Apte MV, Wilson JS, Korsten MA, McCaughan GW, Haber PS,
10 Tolstrup JS, Kristiansen L, Becker U, Grnbaek M. Smoking and Pirola RC. Eects of ethanol and protein deciency on pancreatic
risk of acute and chronic pancreatitis among women and men: a digestive and lysosomal enzymes. Gut 1995; 36: 28793.
population-based cohort study. Arch Intern Med 2009; 169: 60309. 35 Apte MV, Pirola RC, Wilson JS. Where theres smoke theres not
11 Lindkvist B, Appelros S, Manjer J, Berglund G, Borgstrm A. A necessarily re. Gut 2005; 54: 44647.
prospective cohort study of smoking in acute pancreatitis. 36 Pandol SJ, Lugea A, Mareninova OA, et al. Investigating the
Pancreatology 2008; 8: 6370. pathobiology of alcoholic pancreatitis. Alcohol Clin Exp Res 2011;
12 Girman CJ, Kou TD, Cai B, et al. Patients with type 2 diabetes 35: 83037.
mellitus have higher risk for acute pancreatitis compared with 37 Chowdhury P, Walker A. A cell-based approach to study changes in
those without diabetes. Diabetes Obes Metab 2010; 12: 76671. the pancreas following nicotine exposure in an animal model of
13 Urushihara H, Taketsuna M, Liu Y, et al. Increased risk of acute injury. Langenbecks Arch Surg 2008; 393: 54755.
pancreatitis in patients with type 2 diabetes: an observational study 38 Alexandre M, Uduman AK, Minervini S, et al. Tobacco carcinogen
using a Japanese hospital database. PLoS One 2012; 7: e53224. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone initiates and enhances
14 Lai SW, Muo CH, Liao KF, Sung FC, Chen PC. Risk of acute pancreatitis responses. Am J Physiol Gastrointest Liver Physiol 2012;
pancreatitis in type 2 diabetes and risk reduction on anti-diabetic 303: G696704.
drugs: a population-based cohort study in Taiwan. 39 Park CH, Lee IS, Grippo P, Pandol SJ, Gukovskaya AS,
Am J Gastroenterol 2011; 106: 1697704. Edderkaoui M. Akt kinase mediates the prosurvival eect of
15 Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk smoking compounds in pancreatic ductal cells. Pancreas 2013;
of acute pancreatitis and biliary disease observed in patients with 42: 65562.
type 2 diabetes: a retrospective cohort study. Diabetes Care 2009; 40 Rebours V, Vullierme MP, Hentic O, et al. Smoking and the course
32: 83438. of recurrent acute and chronic alcoholic pancreatitis: a
16 Butler PC, Elasho M, Elasho R, Gale EA. A critical analysis of the dose-dependent relationship. Pancreas 2012; 41: 121924.
clinical use of incretin-based therapies: are the GLP-1 therapies 41 Vonlaufen A, Xu Z, Daniel B, et al. Bacterial endotoxin: a trigger
safe? Diabetes Care 2013; 36: 211825. factor for alcoholic pancreatitis? Evidence from a novel,
17 Nauck MA. A critical analysis of the clinical use of incretin-based physiologically relevant animal model. Gastroenterology 2007;
therapies: the benets by far outweigh the potential risks. 133: 1293303.
Diabetes Care 2013; 36: 212632. 42 Whitcomb DC, LaRusch J, Krasinskas AM, et al, and the Alzheimers
18 Bertin C, Pelletier AL, Vullierme MP, et al. Pancreas divisum is not Disease Genetics Consortium. Common genetic variants in the
a cause of pancreatitis by itself but acts as a partner of genetic CLDN2 and PRSS1PRSS2 loci alter risk for alcohol-related and
mutations. Am J Gastroenterol 2012; 107: 31117. sporadic pancreatitis. Nat Genet 2012; 44: 134954.
19 DiMagno MJ, Dimagno EP. Pancreas divisum does not cause 43 Sutton R, Petersen OH, Pandol SJ. Pancreatitis and calcium signalling:
pancreatitis, but associates with CFTR mutations. report of an international workshop. Pancreas 2008; 36: e114.
Am J Gastroenterol 2012; 107: 31820. 44 Frick TW. The role of calcium in acute pancreatitis. Surgery 2012;
20 Dumonceau JM, Andriulli A, Deviere J, et al, and the European 152 (suppl 1): S15763.
Society of Gastrointestinal Endoscopy. European Society of 45 Bradley EL 3rd. A clinically based classication system for acute
Gastrointestinal Endoscopy (ESGE) Guideline: prophylaxis of pancreatitis. Summary of the International Symposium on Acute
post-ERCP pancreatitis. Endoscopy 2010; 42: 50315. Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg
21 Aktas H, Mensink PB, Haringsma J, Kuipers EJ. Low incidence of 1993; 128: 58690.
hyperamylasemia after proximal double-balloon enteroscopy: has 46 Banks PA, Bollen TL, Dervenis C, et al, and the Acute Pancreatitis
the insertion technique improved? Endoscopy 2009; 41: 67073. Classication Working Group. Classication of acute
22 Aktas H, de Ridder L, Haringsma J, Kuipers EJ, Mensink PB. pancreatitis2012: revision of the Atlanta classication and
Complications of single-balloon enteroscopy: a prospective denitions by international consensus. Gut 2013; 62: 10211.
evaluation of 166 procedures. Endoscopy 2010; 42: 36568. 47 Dellinger EP, Forsmark CE, Layer P, et al, and the Pancreatitis
23 Gukovsky I, Pandol SJ, Mareninova OA, Shalbueva N, Jia W, Across Nations Clinical Research and Education Alliance
Gukovskaya AS. Impaired autophagy and organellar dysfunction in (PANCREA). Determinant-based classication of acute pancreatitis
pancreatitis. J Gastroenterol Hepatol 2012; 27 (suppl 2): 2732. severity: an international multidisciplinary consultation. Ann Surg
24 Chiari H. ber die Selbstverdauung des menschlichen Pankreas. 2012; 256: 87580.
Z Heilkunde 1896; 17: 6996 (in German). 48 Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL,
25 Gaisano HY, Lutz MP, Leser J, et al. Supramaximal cholecystokinin Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor
displaces Munc18c from the pancreatic acinar basal surface, of a complex clinical outcome. Crit Care Med 1995; 23: 163852.
redirecting apical exocytosis to the basal membrane. J Clin Invest 49 Lankisch PG, Burchard-Reckert S, Lehnick D. Underestimation of
2001; 108: 1597611. acute pancreatitis: patients with only a small increase in amylase/
26 Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis lipase levels can also have or develop severe acute pancreatitis. Gut
is caused by a mutation in the cationic trypsinogen gene. Nat Genet 1999; 44: 54244.
1996; 14: 14145. 50 Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R,
27 Dawra R, Sah RP, Dudeja V, et al. Intra-acinar trypsinogen Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology
activation mediates early stages of pancreatic injury but not 1985; 156: 76772.
inammation in mice with acute pancreatitis. Gastroenterology 2011; 51 London NJ, Neoptolemos JP, Lavelle J, Bailey I, James D. Contrast-
141: 22102217.e2. enhanced abdominal computed tomography scanning and
28 Capurso G, Zerboni G, Signoretti M, et al. Role of the gut barrier in prediction of severity of acute pancreatitis: a prospective study.
acute pancreatitis. J Clin Gastroenterol 2012; 46 (suppl): S4651. Br J Surg 1989; 76: 26872.
29 Kim JY, Kim KH, Lee JA, et al. Transporter-mediated bile acid 52 King NK, Powell JJ, Redhead D, Siriwardena AK. A simplied
uptake causes Ca+-dependent cell death in rat pancreatic acinar method for computed tomographic estimation of prognosis in acute
cells. Gastroenterology 2002; 122: 194153. pancreatitis. Scand J Gastroenterol 2003; 38: 43336.
94 www.thelancet.com Vol 386 July 4, 2015
Seminar
53 Schrder T, Kivisaari L, Somer K, Standertskjld-Nordenstam CG, 77 Petrov MS, Pylypchuk RD, Uchugina AF. A systematic review on
Kivilaakso E, Lempinen M. Signicance of extrapancreatic ndings the timing of articial nutrition in acute pancreatitis. Br J Nutr
in computed tomography (CT) of acute pancreatitis. Eur J Radiol 2009; 101: 78793.
1985; 5: 27375. 78 Petrov MS, McIlroy K, Grayson L, Phillips AR, Windsor JA. Early
54 De Waele JJ, Delrue L, Hoste EA, De Vos M, Duyck P, Colardyn FA. nasogastric tube feeding versus nil per os in mild to moderate acute
Extrapancreatic inammation on abdominal computed tomography pancreatitis: a randomized controlled trial. Clin Nutr 2013; 32: 697703.
as an early predictor of disease severity in acute pancreatitis: 79 Eatock FC, Chong P, Menezes N, et al. A randomized study of early
evaluation of a new scoring system. Pancreas 2007; 34: 18590. nasogastric versus nasojejunal feeding in severe acute pancreatitis.
55 Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute Am J Gastroenterol 2005; 100: 43239.
pancreatitis: value of CT in establishing prognosis. Radiology 1990; 80 Kumar A, Singh N, Prakash S, Saraya A, Joshi YK. Early enteral
174: 33136. nutrition in severe acute pancreatitis: a prospective randomized
56 Mortele KJ, Wiesner W, Intriere L, et al. A modied CT severity controlled trial comparing nasojejunal and nasogastric routes.
index for evaluating acute pancreatitis: improved correlation with J Clin Gastroenterol 2006; 40: 43134.
patient outcome. AJR Am J Roentgenol 2004; 183: 126165. 81 Singh N, Sharma B, Sharma M, et al. Evaluation of early enteral
57 Bollen TL, Singh VK, Maurer R, et al. A comparative evaluation of feeding through nasogastric and nasojejunal tube in severe acute
radiologic and clinical scoring systems in the early prediction of pancreatitis: a noninferiority randomized controlled trial. Pancreas
severity in acute pancreatitis. Am J Gastroenterol 2012; 107: 61219. 2012; 41: 15359.
58 Spanier BW, Nio Y, van der Hulst RW, Tuynman HA, Dijkgraaf MG, 82 Asrani V, Chang WK, Dong Z, Hardy G, Windsor JA, Petrov MS.
Bruno MJ. Practice and yield of early CT scan in acute pancreatitis: a Glutamine supplementation in acute pancreatitis: a meta-analysis
Dutch observational multicenter study. Pancreatology 2010; 10: 22228. of randomized controlled trials. Pancreatology 2013; 13: 46874.
59 Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing 83 Siriwardena AK, Mason JM, Balachandra S, et al. Randomised,
clinical scoring systems to predict persistent organ failure in patients double blind, placebo controlled trial of intravenous antioxidant
with acute pancreatitis. Gastroenterology 2012; 142: 147682; quiz e1516. (n-acetylcysteine, selenium, vitamin C) therapy in severe acute
60 Lankisch PG, Weber-Dany B, Hebel K, Maisonneuve P, pancreatitis. Gut 2007; 56: 143944.
Lowenfels AB. The harmless acute pancreatitis score: a clinical 84 Mohseni Salehi Monfared SS, Vahidi H, Abdolghaari AH,
algorithm for rapid initial stratication of nonsevere disease. Nikfar S, Abdollahi M. Antioxidant therapy in the management of
Clin Gastroenterol Hepatol 2009; 7: 70205; quiz 607. acute, chronic and post-ERCP pancreatitis: a systematic review.
61 Oskarsson V, Mehrabi M, Orsini N, et al. Validation of the harmless World J Gastroenterol 2009; 15: 448190.
acute pancreatitis score in predicting nonsevere course of acute 85 Bansal D, Bhalla A, Bhasin DK, et al. Safety and ecacy of vitamin-
pancreatitis. Pancreatology 2011; 11: 46468. based antioxidant therapy in patients with severe acute pancreatitis:
62 Talukdar R, Nageshwar Reddy D. Predictors of adverse outcomes in a randomized controlled trial. Saudi J Gastroenterol 2011; 17: 17479.
acute pancreatitis: new horizons. Indian J Gastroenterol 2013; 86 Tse F, Yuan Y. Early routine endoscopic retrograde
32: 14351. cholangiopancreatography strategy versus early conservative
63 Imrie CW, Blumgart LH. Acute pancreatitis: a prospective study on management strategy in acute gallstone pancreatitis.
some factors in mortality. Bull Soc Int Chir 1975; 34: 60103. Cochrane Database Syst Rev 2012; 5: CD009779.
64 Kerner T, Vollmar B, Menger MD, Waldner H, Messmer K. 87 Bai Y, Gao J, Zou DW, Li ZS. Antibiotics prophylaxis in acute
Determinants of pancreatic microcirculation in acute pancreatitis in necrotizing pancreatitis: an update. Am J Gastroenterol 2010; 105: 70507.
rats. J Surg Res 1996; 62: 16571. 88 Wittau M, Mayer B, Scheele J, Henne-Bruns D, Dellinger EP,
65 Haydock MD, Mittal A, Wilms HR, Phillips A, Petrov MS, Isenmann R. Systematic review and meta-analysis of antibiotic
Windsor JA. Fluid therapy in acute pancreatitis: anybodys guess. prophylaxis in severe acute pancreatitis. Scand J Gastroenterol 2011;
Ann Surg 2013; 257: 18288. 46: 26170.
66 Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early uid 89 Jiang K, Huang W, Yang XN, Xia Q. Present and future of
resuscitation reduces morbidity among patients with acute prophylactic antibiotics for severe acute pancreatitis.
pancreatitis. Clin Gastroenterol Hepatol 2011; 9: 70509. World J Gastroenterol 2012; 18: 27984.
67 Mao EQ, Tang YQ, Fei J, et al. Fluid therapy for severe acute 90 Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis
pancreatitis in acute response stage. Chin Med J (Engl) 2009; against infection of pancreatic necrosis in acute pancreatitis.
122: 16973. Cochrane Database Syst Rev 2006; 4: CD002941.
68 de-Madaria E, Soler-Sala G, Snchez-Pay J, et al. Inuence of uid 91 Modi R, Lee AC, Madhavan KK, Garden OJ, Parks RW. Prognostic
therapy on the prognosis of acute pancreatitis: a prospective cohort factors in patients undergoing surgery for severe necrotizing
study. Am J Gastroenterol 2011; 106: 184350. pancreatitis. World J Surg 2007; 31: 200207.
69 Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringers solution 92 Freeman ML, Werner J, van Santvoort HC, et al, and the
reduces systemic inammation compared with saline in patients International Multidisciplinary Panel of Speakers and Moderators.
with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9: 71017; e1. Interventions for necrotizing pancreatitis: summary of a
70 Wu BU, Johannes RS, Sun X, Conwell DL, Banks PA. Early changes multidisciplinary consensus conference. Pancreas 2012; 41: 117694.
in blood urea nitrogen predict mortality in acute pancreatitis. 93 Wittau M, Scheele J, Glz I, Henne-Bruns D, Isenmann R.
Gastroenterology 2009; 137: 12935. Changing role of surgery in necrotizing pancreatitis: a single-center
71 Wu BU, Bakker OJ, Papachristou GI, et al. Blood urea nitrogen in experience. Hepatogastroenterology 2010; 57: 130004.
the early assessment of acute pancreatitis: an international 94 Runzi M, Niebel W, Goebell H, Gerken G, Layer P. Severe acute
validation study. Arch Intern Med 2011; 171: 66976. pancreatitis: nonsurgical treatment of infected necroses. Pancreas
72 Wu BU, Banks PA. Clinical management of patients with acute 2005; 30: 19599.
pancreatitis. Gastroenterology 2013; 144: 127281. 95 van Santvoort HC, Bakker OJ, Bollen TL, et al, and the Dutch
73 Meng W, Yuan J, Zhang C, et al. Parenteral analgesics for pain relief in Pancreatitis Study Group. A conservative and minimally invasive
acute pancreatitis: a systematic review. Pancreatology 2013; 13: 20106. approach to necrotizing pancreatitis improves outcome.
Gastroenterology 2011; 141: 125463.
74 American Society of Anesthesiologists Task Force on Acute Pain
Management. Practice guidelines for acute pain management in the 96 Seifert H, Wehrmann T, Schmitt T, Zeuzem S, Caspary WF.
perioperative setting: an updated report by the American Society of Retroperitoneal endoscopic debridement for infected peripancreatic
Anesthesiologists Task Force on Acute Pain Management. necrosis. Lancet 2000; 356: 65355.
Anesthesiology 2012; 116: 24873. 97 Seifert H, Biermer M, Schmitt W, et al. Transluminal endoscopic
75 Singla A, Simons J, Li Y, et al. Admission volume determines necrosectomy after acute pancreatitis: a multicentre study with
outcome for patients with acute pancreatitis. Gastroenterology 2009; long-term follow-up (the GEPARD Study). Gut 2009; 58: 126066.
137: 19952001. 98 Bakker OJ, van Santvoort HC, van Brunschot S, et al, and the Dutch
76 Petrov MS, Pylypchuk RD, Emelyanov NV. Systematic review: Pancreatitis Study Group. Endoscopic transgastric vs surgical
nutritional support in acute pancreatitis. Aliment Pharmacol Ther necrosectomy for infected necrotizing pancreatitis: a randomized
2008; 28: 70412. trial. JAMA 2012; 307: 105361.
www.thelancet.com Vol 386 July 4, 2015 95
Seminar
99 van Santvoort HC, Besselink MG, Bakker OJ, et al, and the Dutch 118 Mujica VR, Barkin JS, Go VL, and the Study Group Participants.
Pancreatitis Study Group. A step-up approach or open Acute pancreatitis secondary to pancreatic carcinoma. Pancreas
necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 2000; 21: 32932.
362: 1491502. 119 Wu D, Xu Y, Zeng Y, Wang X. Endocrine pancreatic function
100 Gardner TB, Coelho-Prabhu N, Gordon SR, et al. Direct endoscopic changes after acute pancreatitis. Pancreas 2011; 40: 100611.
necrosectomy for the treatment of walled-o pancreatic necrosis: 120 Pezzilli R, Simoni P, Casadei R, Morselli-Labate AM. Exocrine
results from a multicenter U.S. series. Gastrointest Endosc 2011; pancreatic function during the early recovery phase of acute
73: 71826. pancreatitis. Hepatobiliary Pancreat Dis Int 2009; 8: 31619.
101 Lankisch PG, Weber-Dany B, Maisonneuve P, Lowenfels AB. 121 Symersky T, van Hoorn B, Masclee AA. The outcome of a long-term
Pancreatic pseudocysts: prognostic factors for their development follow-up of pancreatic function after recovery from acute
and their spontaneous resolution in the setting of acute pancreatitis. JOP 2006; 7: 44753.
pancreatitis. Pancreatology 2012; 12: 8590. 122 Doepel M, Eriksson J, Halme L, Kumpulainen T, Hckerstedt K.
102 Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Good long-term results in patients surviving severe acute
Wilcox CM. Prospective randomized trial comparing EUS and EGD pancreatitis. Br J Surg 1993; 80: 158386.
for transmural drainage of pancreatic pseudocysts (with videos). 123 Lankisch PG, Breuer N, Bruns A, Weber-Dany B, Lowenfels AB,
Gastrointest Endosc 2008; 68: 110211. Maisonneuve P. Natural history of acute pancreatitis: a long-term
103 Varadarajulu S, Noone TC, Tutuian R, Hawes RH, Cotton PB. population-based study. Am J Gastroenterol 2009;
Predictors of outcome in pancreatic duct disruption managed by 104: 2797805; quiz 2806.
endoscopic transpapillary stent placement. Gastrointest Endosc 2005; 124 Njgaard C, Becker U, Matzen P, Andersen JR, Holst C,
61: 56875. Bendtsen F. Progression from acute to chronic pancreatitis:
104 Lawrence C, Howell DA, Stefan AM, et al. Disconnected pancreatic prognostic factors, mortality, and natural course. Pancreas 2011;
tail syndrome: potential for endoscopic therapy and results of 40: 1195200.
long-term follow-up. Gastrointest Endosc 2008; 67: 67379. 125 Yadav D, OConnell M, Papachristou GI. Natural history following
105 Butler JR, Eckert GJ, Zyromski NJ, Leonardi MJ, Lillemoe KD, the rst attack of acute pancreatitis. Am J Gastroenterol 2012;
Howard TJ. Natural history of pancreatitis-induced splenic vein 107: 1096103.
thrombosis: a systematic review and meta-analysis of its incidence 126 Seidensticker F, Otto J, Lankisch PG. Recovery of the pancreas after
and rate of gastrointestinal bleeding. HPB (Oxford) 2011; 13: 83945. acute pancreatitis is not necessarily complete. Int J Pancreatol 1995;
106 Bergert H, Hinterseher I, Kersting S, Leonhardt J, Bloomenthal A, 17: 22529.
Saeger HD. Management and outcome of hemorrhage due to 127 Nordback I, Pelli H, Lappalainen-Lehto R, Jrvinen S, Rty S,
arterial pseudoaneurysms in pancreatitis. Surgery 2005; 137: 32328. Sand J. The recurrence of acute alcohol-associated pancreatitis can
107 Balachandra S, Siriwardena AK. Systematic appraisal of the be reduced: a randomized controlled trial. Gastroenterology 2009;
management of the major vascular complications of pancreatitis. 136: 84855.
Am J Surg 2005; 190: 48995. 128 Bakker OJ, van Santvoort HC, Hagenaars JC, et al, for the Dutch
108 Besselink MG, van Santvoort HC, Boermeester MA, et al, and the Pancreatitis Study Group. Timing of cholecystectomy after mild
Dutch Acute Pancreatitis Study Group. Timing and impact of biliary pancreatitis Br J Surg 2011; 98: 144654.
infections in acute pancreatitis. Br J Surg 2009; 96: 26773. 129 Mazaki T, Mado K, Masuda H, Shiono M. Prophylactic pancreatic
109 Wu BU, Johannes RS, Kurtz S, Banks PA. The impact of hospital- stent placement and post-ERCP pancreatitis: an updated
acquired infection on outcome in acute pancreatitis. meta-analysis. J Gastroenterol 2013; 49: 34355.
Gastroenterology 2008; 135: 81620. 130 Cennamo V, Fuccio L, Zagari RM, et al. Can early precut
110 Berzin TM, Rocha FG, Whang EE, Mortele KJ, Ashley SW, implementation reduce endoscopic retrograde
Banks PA. Prevalence of primary fungal infections in necrotizing cholangiopancreatography-related complication risk? Meta-analysis
pancreatitis. Pancreatology 2007; 7: 6366. of randomized controlled trials. Endoscopy 2010; 42: 38188.
111 Meier R, Ockenga J, Pertkiewicz M, et al, and the DGEM (German 131 Lankisch PG, Koop H, Winckler K, Kunze H, Vogt W. Indomethacin
Society for Nutritional Medicine), and the ESPEN (European Society treatment of acute experimental pancreatitis in the rat.
for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral Scand J Gastroenterol 1978; 13: 62933.
nutrition: pancreas. Clin Nutr 2006; 25: 27584. 132 Ebbehj N, Friis J, Svendsen LB, Blow S, Madsen P. Indomethacin
112 Teich N, Aghdassi A, Fischer J, et al. Optimal timing of oral treatment of acute pancreatitis. A controlled double-blind trial.
refeeding in mild acute pancreatitis: results of an open randomized Scand J Gastroenterol 1985; 20: 798800.
multicenter trial. Pancreas 2010; 39: 108892. 133 Dai HF, Wang XW, Zhao K. Role of nonsteroidal anti-inammatory
113 Li J, Xue GJ, Liu YL, et al. Early oral refeeding wisdom in patients drugs in the prevention of post-ERCP pancreatitis: a meta-analysis.
with mild acute pancreatitis. Pancreas 2013; 42: 8891. Hepatobiliary Pancreat Dis Int 2009; 8: 1116.
114 Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric 134 Elmunzer BJ, Scheiman JM, Lehman GA, et al, and the U.S.
feeding in predicted severe acute pancreatitis: a clinical, Cooperative for Outcomes Research in Endoscopy (USCORE).
randomized study. Ann Surg 2006; 244: 95965; discussion 96567. A randomized trial of rectal indomethacin to prevent post-ERCP
115 Sathiaraj E, Murthy S, Mansard MJ, Rao GV, Mahukar S, Reddy DN. pancreatitis. N Engl J Med 2012; 366: 141422.
Clinical trial: oral feeding with a soft diet compared with clear liquid 135 Zheng MH, Xia HH, Chen YP. Rectal administration of NSAIDs in
diet as initial meal in mild acute pancreatitis. the prevention of post-ERCP pancreatitis: a complementary
Aliment Pharmacol Ther 2008; 28: 77781. meta-analysis. Gut 2008; 57: 163233.
116 Jacobson BC, Vander Vliet MB, Hughes MD, Maurer R, 136 Akbar A, Abu Dayyeh BK, Baron TH, Wang Z, Altayar O,
McManus K, Banks PA. A prospective, randomized trial of clear Murad MH. Rectal nonsteroidal anti-inammatory drugs are
liquids versus low-fat solid diet as the initial meal in mild acute superior to pancreatic duct stents in preventing pancreatitis after
pancreatitis. Clin Gastroenterol Hepatol 2007; 5: 94651; quiz 886. endoscopic retrograde cholangiopancreatography: a network
117 Moraes JM, Felga GE, Chebli LA, et al. A full solid diet as the initial meta-analysis. Clin Gastroenterol Hepatol 2013; 11: 77883.
meal in mild acute pancreatitis is safe and result in a shorter length of 137 Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring
hospitalization: results from a prospective, randomized, controlled, role in normal and diseased pancreas. Front Physiol 2012; 3: 344.
double-blind clinical trial. J Clin Gastroenterol 2010; 44: 51722.
96 www.thelancet.com Vol 386 July 4, 2015
You might also like
- Pancretitis AgudaDocument12 pagesPancretitis Agudapruebaprueba321765No ratings yet
- Pancreatitis AgudaDocument12 pagesPancreatitis AgudaWILLIAM RICARDO CASTAÑEDA VARGASNo ratings yet
- 8a Acute PancreatitisDocument12 pages8a Acute PancreatitisTony SswwNo ratings yet
- Acute Pancreatitis Lancet 2015Document12 pagesAcute Pancreatitis Lancet 2015dianakstNo ratings yet
- Acute Pancreatitis 2015Document12 pagesAcute Pancreatitis 2015Mayra Alejandra Prada SerranoNo ratings yet
- Pancreatitis Aguda para Emergencia PDFDocument11 pagesPancreatitis Aguda para Emergencia PDFJudithzaloNo ratings yet
- Global Epidemiology and Holistic Prevention of PancreatitisDocument24 pagesGlobal Epidemiology and Holistic Prevention of PancreatitisRoberto MaresNo ratings yet
- Acute Pancreatitis (Annals of IM)Document16 pagesAcute Pancreatitis (Annals of IM)Jorge Armando Arrieta GalvanNo ratings yet
- Reporting of Acute PancreatitisDocument13 pagesReporting of Acute PancreatitisThanh NguyenNo ratings yet
- Acute Pancreatitis. Lancet Seminar Sept 5, 2020Document9 pagesAcute Pancreatitis. Lancet Seminar Sept 5, 2020Luis Henrique SalesNo ratings yet
- 1 s2.0 S0140673620313106 MainDocument9 pages1 s2.0 S0140673620313106 MainEdwin Fabian Usaquen KairuzNo ratings yet
- Pancreatitis PDFDocument10 pagesPancreatitis PDFEmilio' 'BatistaNo ratings yet
- In The Clinic - Acute PancreatitisDocument16 pagesIn The Clinic - Acute PancreatitisSurapon Nochaiwong100% (1)
- Acute Pancreatitis: Current Perspectives On Diagnosis and ManagementDocument9 pagesAcute Pancreatitis: Current Perspectives On Diagnosis and ManagementGita Septiyani KusumaNo ratings yet
- NCDD 04272009 ResearchPlan DiseasesofPancreasDocument10 pagesNCDD 04272009 ResearchPlan DiseasesofPancreasGmail JONo ratings yet
- New Insights Into Acute Pancreatitis: Peter J. Lee and Georgios I. PapachristouDocument18 pagesNew Insights Into Acute Pancreatitis: Peter J. Lee and Georgios I. PapachristouandreaNo ratings yet
- Acute PancreatitisDocument9 pagesAcute PancreatitispreetshardocNo ratings yet
- Braganza2011 PDFDocument14 pagesBraganza2011 PDFAmirullah AbdiNo ratings yet
- New Insights of Acute PancreatitisDocument18 pagesNew Insights of Acute Pancreatitispruebaprueba321765No ratings yet
- 10 1056@NEJMra1505202 PDFDocument10 pages10 1056@NEJMra1505202 PDFFiorella ChambiNo ratings yet
- Chronic PancreatitisDocument7 pagesChronic PancreatitisMr. LNo ratings yet
- Acute PancreatitisDocument10 pagesAcute PancreatitisAndrés Menéndez RojasNo ratings yet
- Etiology PDFDocument22 pagesEtiology PDFdrwagnerzaoNo ratings yet
- Acute Pancreatitis: Clinical PR ActiceDocument9 pagesAcute Pancreatitis: Clinical PR ActicesanthiagoschneiderNo ratings yet
- Acute PancreatitsiDocument8 pagesAcute PancreatitsiAnty FftNo ratings yet
- Acute Pancreatitis Review and Clinical UpdateDocument10 pagesAcute Pancreatitis Review and Clinical Updateemanuel santiago camargo parraNo ratings yet
- Acute Pancreatitis: in The ClinicDocument16 pagesAcute Pancreatitis: in The ClinicdeltanueveNo ratings yet
- Management of Severe Acute PancreatitisDocument12 pagesManagement of Severe Acute PancreatitisSusan Yanapa ZelaNo ratings yet
- Manejo de PancreatitisDocument12 pagesManejo de Pancreatitiskarina hernandezNo ratings yet
- Pancreatitis DRDocument12 pagesPancreatitis DRAlexis TobarNo ratings yet
- Pankreatitis 2017Document13 pagesPankreatitis 2017GitarefinaNo ratings yet
- Management of Severe Acute PancreatitisDocument12 pagesManagement of Severe Acute PancreatitiszajosNo ratings yet
- Guia PancreasDocument11 pagesGuia PancreasYusleimin MartinezNo ratings yet
- JAMA 2021 Acute Pancreatitis. A ReviewDocument9 pagesJAMA 2021 Acute Pancreatitis. A ReviewPaloma GB100% (3)
- Wang 2009Document4 pagesWang 2009DANY ALEXIS SOBARZO SOTONo ratings yet
- Acute Pancreatitis Diagnosis and TreatmentDocument27 pagesAcute Pancreatitis Diagnosis and TreatmentkarlosNo ratings yet
- Factors Leading to Acute Pancreatitis in PakistanDocument4 pagesFactors Leading to Acute Pancreatitis in Pakistansf228No ratings yet
- The Revised Atlanta Classification For Acute Pancreatitis: Updates in Imaging Terminology and GuidelinesDocument12 pagesThe Revised Atlanta Classification For Acute Pancreatitis: Updates in Imaging Terminology and Guidelinesjaider luis saurith monterrosaNo ratings yet
- Acute PancreatitisDocument12 pagesAcute PancreatitisBÁCH NGUYỄN ĐẮCNo ratings yet
- Ten Major Concerns About Antibiotic Use in Acute PancreatitisDocument9 pagesTen Major Concerns About Antibiotic Use in Acute PancreatitislakshitNo ratings yet
- Fast Facts: Complex Perianal Fistulas in Crohn's Disease: A multidisciplinary approach to a clinical challengeFrom EverandFast Facts: Complex Perianal Fistulas in Crohn's Disease: A multidisciplinary approach to a clinical challengeNo ratings yet
- Management of Infected Pancreatic Necrosis: State of The ArtDocument9 pagesManagement of Infected Pancreatic Necrosis: State of The ArtSares DaselvaNo ratings yet
- Jurnal: Acute PancreatitisDocument11 pagesJurnal: Acute Pancreatitisahmad fachryNo ratings yet
- Pleural Effusion Predicts Severity in Acute PancreatitisDocument4 pagesPleural Effusion Predicts Severity in Acute PancreatitiszajosNo ratings yet
- An Observational Study To Determine Relation Between Various Indices of Acute PancreatitisDocument4 pagesAn Observational Study To Determine Relation Between Various Indices of Acute PancreatitisDr. NasrumminallahNo ratings yet
- Jurnal DhaDocument11 pagesJurnal DhasiydahNo ratings yet
- 2016 Article 1292 PDFDocument10 pages2016 Article 1292 PDFPutriRahayuMoidadyNo ratings yet
- JCTH-7-154Document11 pagesJCTH-7-154Andreea AlexandruNo ratings yet
- Pancreatitis RevDocument14 pagesPancreatitis Revbmoreno78No ratings yet
- Pharmacologic Management and Prevention of Acute PancreatitisDocument8 pagesPharmacologic Management and Prevention of Acute PancreatitisarnoldNo ratings yet
- Clinical-Liver, Pancreas, and Biliary TractDocument8 pagesClinical-Liver, Pancreas, and Biliary TractKirsten HartmanNo ratings yet
- Renal Disease in Cancer PatientsFrom EverandRenal Disease in Cancer PatientsKevin W. FinkelNo ratings yet
- Diseases of the Liver and Biliary TreeFrom EverandDiseases of the Liver and Biliary TreeAnnarosa FloreaniNo ratings yet
- Upper Tract Urothelial CarcinomaFrom EverandUpper Tract Urothelial CarcinomaShahrokh F. ShariatNo ratings yet
- Variceal HemorrhageFrom EverandVariceal HemorrhageRoberto de FranchisNo ratings yet
- Esophageal Cancer: Diagnosis and TreatmentFrom EverandEsophageal Cancer: Diagnosis and TreatmentFrancisco SchlottmannNo ratings yet
- Acute Gastrointestinal Bleeding: Diagnosis and TreatmentFrom EverandAcute Gastrointestinal Bleeding: Diagnosis and TreatmentKaren E. KimNo ratings yet
- Secondary HypertensionFrom EverandSecondary HypertensionAlberto MorgantiNo ratings yet
- DyslipidemiaDocument87 pagesDyslipidemiaCarlos Arturo Paternina Cuello100% (1)
- DyslipidemiaDocument87 pagesDyslipidemiaCarlos Arturo Paternina Cuello100% (1)
- Contemporary Reviews in Cardiovascular Medicine: Recent Update To The US Cholesterol Treatment GuidelinesDocument25 pagesContemporary Reviews in Cardiovascular Medicine: Recent Update To The US Cholesterol Treatment GuidelinesKaren JboNo ratings yet
- Pi Is 0828282 X 16307322Document20 pagesPi Is 0828282 X 16307322Karen JboNo ratings yet
- ENPancreas PDFDocument10 pagesENPancreas PDFTania Sanguña RonNo ratings yet
- Consenso Italiano PA SeveraDocument70 pagesConsenso Italiano PA SeveraFabrizzio BardalesNo ratings yet
- ATP III Guideline KolesterolDocument6 pagesATP III Guideline KolesterolRakasiwi GalihNo ratings yet
- Opportunistic InfectionsDocument430 pagesOpportunistic InfectionsKaren JboNo ratings yet
- New ERCP Classification System Predicts Diagnoses and Adverse EventsDocument10 pagesNew ERCP Classification System Predicts Diagnoses and Adverse EventsKaren JboNo ratings yet
- ZJCH 7 1361292 - JDocument6 pagesZJCH 7 1361292 - JKaren JboNo ratings yet
- ESPEN Guidelines On Nutrition in Acute Pancreatitis: Consensus StatementDocument11 pagesESPEN Guidelines On Nutrition in Acute Pancreatitis: Consensus StatementMemo HadyNo ratings yet
- WJG 20 16123 PDFDocument10 pagesWJG 20 16123 PDFKaren JboNo ratings yet
- The Potential Role of Gut Microbiota in Pancreatic Disease: A Systematic ReviewDocument2 pagesThe Potential Role of Gut Microbiota in Pancreatic Disease: A Systematic ReviewKaren JboNo ratings yet
- 10.7326@acpjc 2017 167 8 044Document1 page10.7326@acpjc 2017 167 8 044Karen JboNo ratings yet
- Piis1424390313005255 PDFDocument15 pagesPiis1424390313005255 PDFKaren JboNo ratings yet
- Final Meta IdfDocument24 pagesFinal Meta IdfdoublejaydNo ratings yet
- Dealing with Difficult BehaviorDocument63 pagesDealing with Difficult BehaviorKaren JboNo ratings yet
- Dyslipidaemias 2016 2016-08Document74 pagesDyslipidaemias 2016 2016-08Karen JboNo ratings yet
- Bariatric Surgery: Postoperative Nutritional Management - UpToDateDocument32 pagesBariatric Surgery: Postoperative Nutritional Management - UpToDateKaren Jbo100% (1)
- Drinking Water and Losing WeightDocument3 pagesDrinking Water and Losing WeightMathana SuriaNo ratings yet
- The Psychiatric Comorbidity of Epilepsy PDFDocument14 pagesThe Psychiatric Comorbidity of Epilepsy PDFTanjung E SumekarNo ratings yet
- Effects of Hypoxia on Health ConditionsDocument36 pagesEffects of Hypoxia on Health ConditionsVeres András100% (2)
- CPG Labor and Delivery 2015Document78 pagesCPG Labor and Delivery 2015TintalleNo ratings yet
- Stanford Hospital & Clinics Antimicrobial Dosing Reference Guide 2013Document3 pagesStanford Hospital & Clinics Antimicrobial Dosing Reference Guide 2013SANCHOSKYNo ratings yet
- Medicine CNS-ENDODocument11 pagesMedicine CNS-ENDOmrcopy xeroxNo ratings yet
- Aids HandoutDocument2 pagesAids Handoutapi-272957818No ratings yet
- Status EpilepticusDocument22 pagesStatus EpilepticusVivi Kristiani RumapeaNo ratings yet
- REBOZETDocument28 pagesREBOZETAndronikus DharmawanNo ratings yet
- Dermatology Chap6. Bacterial Infections of Skin 2020-3-23Document368 pagesDermatology Chap6. Bacterial Infections of Skin 2020-3-23Mohsin Tanmoy100% (1)
- Education: Recreational TherapistDocument3 pagesEducation: Recreational Therapistapi-570783384No ratings yet
- DFSGDFGDFDocument60 pagesDFSGDFGDFMada Dwi HariNo ratings yet
- Stool Exam Guide - Key Tests, Colors, Consistencies in 40 CharactersDocument8 pagesStool Exam Guide - Key Tests, Colors, Consistencies in 40 CharactersLester John HilarioNo ratings yet
- Doctor's OrderDocument2 pagesDoctor's OrderWiljohn de la CruzNo ratings yet
- Emrcp CNS 38-62Document22 pagesEmrcp CNS 38-62dryusufsNo ratings yet
- Recent Updates On Nanomedicine Based Products: Current Sce-Nario and Future OpportunitiesDocument13 pagesRecent Updates On Nanomedicine Based Products: Current Sce-Nario and Future OpportunitiesvijuNo ratings yet
- Commonly Used Terminologies in Fish Disease: Jitendra Lama Roll No: 16Document4 pagesCommonly Used Terminologies in Fish Disease: Jitendra Lama Roll No: 16Santosh BhandariNo ratings yet
- Early Intensive Care Unit Mobility Therapy in The Treatment of Acute Respiratory FailureDocument6 pagesEarly Intensive Care Unit Mobility Therapy in The Treatment of Acute Respiratory FailureTakashi NakamuraNo ratings yet
- Urea Cycle DefectsDocument12 pagesUrea Cycle DefectsAleksandra RadonjićNo ratings yet
- Ors, Hyd Mix& Road To H CardDocument10 pagesOrs, Hyd Mix& Road To H CardAnonymous hYMWbANo ratings yet
- Gordon's 11 Functional Health PatternDocument2 pagesGordon's 11 Functional Health PatternWendell AcuñaNo ratings yet
- Books Shalya TantraDocument4 pagesBooks Shalya Tantrajmanthan56No ratings yet
- PhenobarbitalDocument2 pagesPhenobarbitalhahahahaaaaaaaNo ratings yet
- Physical Health and Mental HealthDocument2 pagesPhysical Health and Mental HealthLuise MauieNo ratings yet
- Characteristics of Functional Shoulder InstabilityDocument11 pagesCharacteristics of Functional Shoulder InstabilityOla BochniakNo ratings yet
- Dialog Diabetes B.inggris Kel 5-1Document4 pagesDialog Diabetes B.inggris Kel 5-1Desma LindaNo ratings yet
- Anorganik Chemistry 3Document5 pagesAnorganik Chemistry 3putrik agustinaNo ratings yet
- Fetal DistressDocument49 pagesFetal DistressLenny SucalditoNo ratings yet
- Basic Pharmacovigilance Interview Questions For FRESHERSDocument8 pagesBasic Pharmacovigilance Interview Questions For FRESHERSPrakash MishraNo ratings yet
- BBMS FeaturesDocument1 pageBBMS FeaturesVic McknightNo ratings yet