Professional Documents
Culture Documents
Van TH Off Plots
Uploaded by
hmtaad114Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Van TH Off Plots
Uploaded by
hmtaad114Copyright:
Available Formats
Biochemistry – Van’t Hoff plots and protein folding.
Consider a protein and a single point mutation in two states, native (folded) and denatured (unfolded).
Native ó Denatured.
The equilibrium is defined like any other reaction: Keq = [denatured protein] / [native protein]
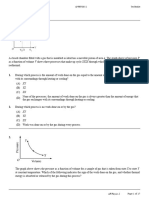
If one uses plane polarized light or fluorescence to Protein
melting
–
Transition
Curve
determine the fraction of protein folded or unfolded at 1.0
Fraction of unfolded Protein
different temps a protein-melting curve (see graph) Ala 54 mutation
can be produced. (red dashed line)
Both the Enthalpy (ΔH) and entropy (ΔS) can be
calculated using a curve of a fraction (%) of protein in 0.5
folded and unfolded state. The S and H values can be Tm = 50%
calculated using the van’t Hoff equation and plot. The folded/unfolded
Van’t Hoff equation informs about the temperature Thr 54 Wild-‐type
dependence of the equilibrium constant. (blue solid line)
0.0
** To use this equation, the protein must refold – this is
an equilibrium problem! 10 50 90
Temp
Tm = 69oC (342K)
The van’t Hoff equation is derived from Gives Free
energy equation: ΔG = ΔH – T ΔS
Then also understanding that for this reaction of folding and unfolding: ΔG= -RT lnKeq
We can create the van’t Hoff equation to relate equilibrium constant to
temperature by substituting the two equations and rearranging to generate
van’t Hoff Plot
the van’t Hoff equation:
lnKeq = - ΔH/R(1/T) + ΔS/R
Note: The equation is a straight line Y=mx+B. Therefore a plot of lnKeq vs
1/T, known as the van’t Hoff plot, yields a straight line of slope -ΔH/RT and
Y intercept = ΔS/R.
Using both the melting or transition curve and Van’t Hoff’s plot and
equation, we can determine the thermodynamic functions of protein
stability (the fraction of protein at a given temperature that is native or denatured).
• Determine the fraction of protein folded from the transition curve where [native protein] = [denatured protein]
and convert that information to Keq for each temperature.
• From this data you can create a van’t Hoff plot and…
• Calculate the ΔH and ΔS from the slope and Y intercept.
Ex – for our wild-type protein, determine G, S an H.
1. Create a van’t Hoff plot - Start by using the conversion of the transition graph to a van’t Hoff plot
2. Calculate ΔH. The slope of van’t Hoff plot will give: ΔH
for a test you would be given this information or the slope and intercept of a van’t Hoff plot).
3. Calculate ΔG: Using the melting point (Tm) to determine ΔG
a. Keq = [denatured protein] / [native protein] = 1.0. AND
b. ΔG= -RT lnKeq because, ln1.0 is = 0.0. Therefore, at the Tm ΔG= 0
4. Calculate ΔS: Again, using and rearranging the van’t Hoff plot/equation: ΔS = ΔH/Tm
5. Now you have ΔS and ΔH for this protein and can determine the protein stability at any other temperature
using ΔG = ΔH – T ΔS
To determine the stability of a protein with and without some change (ligand binding, protein interaction or mutation),
determine the ΔG for each protein ΔΔG = ΔG wild-type - ΔG mutant. A POSTIVE value indicates that the unfolding of
the wild type is LESS favorable than the mutant by the calculated value (that the mutation decreases the stability of
the native protein). A NEGATIVE value indicates the unfolding of the wild type is More favorable than the mutant (or
that the mutation stabilizes he native structure)
You might also like
- Part 3Document25 pagesPart 3Zyber ColcolNo ratings yet
- CPE 201 Module 4Document7 pagesCPE 201 Module 4Nurudeen OlumegbonNo ratings yet
- Publication 2 21263 432Document4 pagesPublication 2 21263 432Andre MuhammadNo ratings yet
- Van 'T Hoff EquationDocument36 pagesVan 'T Hoff EquationPraveen KumarNo ratings yet
- Chapter 12 - Thermodynamic Property RelationsDocument27 pagesChapter 12 - Thermodynamic Property RelationsNurshaqina SufianNo ratings yet
- Critical Exponents - Chemical Reactions and Chemical Equilibrium - Thermodynamics in Other SettingsDocument16 pagesCritical Exponents - Chemical Reactions and Chemical Equilibrium - Thermodynamics in Other SettingsnokosamNo ratings yet
- 3MN110 Landau Theory Exam 2022-1Document4 pages3MN110 Landau Theory Exam 2022-1snpttpkoqvzhnmskbtNo ratings yet
- Estimation of Activation Energy by Isoconversion Methods PDFDocument7 pagesEstimation of Activation Energy by Isoconversion Methods PDFnonameNo ratings yet
- Solidification: T S, While For Processes at Constant Volume V T S, Where H and U Are TheDocument21 pagesSolidification: T S, While For Processes at Constant Volume V T S, Where H and U Are TheClaudia Belén Cortés RuizNo ratings yet
- CY-008 Lecture-III&IVDocument34 pagesCY-008 Lecture-III&IVakashNo ratings yet
- Thermodynamic RelationsDocument28 pagesThermodynamic RelationsAmrita DinilNo ratings yet
- Phase Separation Formation of Solid Solution Compound FormationDocument12 pagesPhase Separation Formation of Solid Solution Compound FormationAkashNo ratings yet
- Hermodynamics of Phase Change: Hase ChangesDocument14 pagesHermodynamics of Phase Change: Hase ChangesdemirciNo ratings yet
- Transient Conduction Analysis The Lumped Capacitance Method: Class #6Document15 pagesTransient Conduction Analysis The Lumped Capacitance Method: Class #6Valentina Mejia GallonNo ratings yet
- 20 Van Der Waals Model of Liquid-Gas Phase TransitionDocument7 pages20 Van Der Waals Model of Liquid-Gas Phase TransitionAlejandro RMNo ratings yet
- Activity in InfiniteDocument5 pagesActivity in InfiniteLelis Clemente FigueroaNo ratings yet
- Phase Equillibria-VrazDocument46 pagesPhase Equillibria-VrazSindhu KemburuNo ratings yet
- 2022 CIR310 Sem Test 2 With AnswersDocument9 pages2022 CIR310 Sem Test 2 With Answersu21589969No ratings yet
- Shav It 22 DomainDocument6 pagesShav It 22 DomainwhateverNo ratings yet
- ME 267: Advanced Thermodynamics I: Thermodynamic RelationsDocument73 pagesME 267: Advanced Thermodynamics I: Thermodynamic Relationsluzviminda ramosNo ratings yet
- Heat Capacity: at Coun..pDocument4 pagesHeat Capacity: at Coun..pMunikumar KandulaNo ratings yet
- Thermodynamics: Properties of Single-Component Systems NomenclatureDocument11 pagesThermodynamics: Properties of Single-Component Systems Nomenclaturevzimak2355No ratings yet
- Design of A Vertical Flash Drum-ReportDocument6 pagesDesign of A Vertical Flash Drum-ReportSehry SyedNo ratings yet
- Thermodynamic Property RelationsDocument26 pagesThermodynamic Property RelationsSec CNo ratings yet
- Prater Vol 8 284 1958Document3 pagesPrater Vol 8 284 1958Dai DomNo ratings yet
- APC - Chapter 5 - Part 1 SP22Document20 pagesAPC - Chapter 5 - Part 1 SP22iB13eNo ratings yet
- THERMO10 - Thermodynamics of Phase DiagramsDocument8 pagesTHERMO10 - Thermodynamics of Phase DiagramsBayu Setio RaharjoNo ratings yet
- Enthalpy: H U PV H U+PV TP UDocument3 pagesEnthalpy: H U PV H U+PV TP UAnshul TeotiaNo ratings yet
- Experiment 6 Vapor Pressure of Pure LiquidsDocument8 pagesExperiment 6 Vapor Pressure of Pure LiquidsHaNo ratings yet
- CH 6Document30 pagesCH 6tamay 95No ratings yet
- Phase Transitions in Field Theories at Finite Temperatures: 1 MotivationDocument5 pagesPhase Transitions in Field Theories at Finite Temperatures: 1 MotivationchichieinsteinNo ratings yet
- Closed Systems: 1 Boundary WorkDocument2 pagesClosed Systems: 1 Boundary Workwee rrNo ratings yet
- Review Phase Equilibrium and VLE ModelingDocument65 pagesReview Phase Equilibrium and VLE ModelingW_LinNo ratings yet
- Vapor/Liquid Equilibrium: Mata Kuliah: Termodinamika IIDocument70 pagesVapor/Liquid Equilibrium: Mata Kuliah: Termodinamika IIputri wahyuniNo ratings yet
- Ii 4Document17 pagesIi 4Paul Barragán GómezNo ratings yet
- PDF PDFDocument25 pagesPDF PDFelbronNo ratings yet
- CL 208 Chemical Reaction Engineering-IDocument34 pagesCL 208 Chemical Reaction Engineering-ISatkar JainNo ratings yet
- Lecture 1Document31 pagesLecture 1Nimit RiniNo ratings yet
- Collapse of SupersymmetryDocument22 pagesCollapse of SupersymmetrySantosh SinghNo ratings yet
- OP Flash DistillationDocument11 pagesOP Flash DistillationDylan Navarro LNo ratings yet
- Arrhenius EquationDocument10 pagesArrhenius EquationRavinder KaurNo ratings yet
- Latent Heat of Vaporization of EthanolDocument5 pagesLatent Heat of Vaporization of EthanolMel DyNo ratings yet
- HX NotesDocument17 pagesHX Notesjuwel KabirNo ratings yet
- Cmat Term Paper: 1 Phase Space and EnsemblesDocument5 pagesCmat Term Paper: 1 Phase Space and EnsemblesiamrohanNo ratings yet
- Chapter 3Document26 pagesChapter 3BISRAT YIHUNNo ratings yet
- Thermodynamic Revision 2021 Part 1Document70 pagesThermodynamic Revision 2021 Part 1Rawda AliNo ratings yet
- Computing Solubility Parameters of Deep Eutectic Solvents From Molecular Dynamics SimulationsDocument27 pagesComputing Solubility Parameters of Deep Eutectic Solvents From Molecular Dynamics SimulationsStalwart sheikhNo ratings yet
- Capítulo 6Document85 pagesCapítulo 6Lorena VivasNo ratings yet
- Clap 2Document14 pagesClap 2api-285318286No ratings yet
- Phase Equilibrium For Separator Design - 0Document52 pagesPhase Equilibrium For Separator Design - 0May May MagluyanNo ratings yet
- 1-Vle Part 1Document30 pages1-Vle Part 1Arfa Zulkifli01No ratings yet
- Enthalpy vs. Composition - Ponchon-Savarit PlotDocument32 pagesEnthalpy vs. Composition - Ponchon-Savarit PlotahmedNo ratings yet
- ER CH3-Student 2022Document45 pagesER CH3-Student 2022aboem16No ratings yet
- ثرمو2Document19 pagesثرمو2Al-Hassan NeimaNo ratings yet
- Multiphase Systems - Part IDocument20 pagesMultiphase Systems - Part I랄뚜기No ratings yet
- The Maxwell Relations: CHEM 331 Physical Chemistry Fall 2017Document11 pagesThe Maxwell Relations: CHEM 331 Physical Chemistry Fall 2017AhmedovicMichaelNo ratings yet
- Ejercicios Boze Einstein PDFDocument17 pagesEjercicios Boze Einstein PDFkaren maria camino galvezNo ratings yet
- GPSA Propiedades Termodinamicas 24 PDFDocument42 pagesGPSA Propiedades Termodinamicas 24 PDFDavid Cortez PeraltaNo ratings yet
- Hadamard Transform: Unveiling the Power of Hadamard Transform in Computer VisionFrom EverandHadamard Transform: Unveiling the Power of Hadamard Transform in Computer VisionNo ratings yet
- A History of PhotographyDocument49 pagesA History of PhotographyderghalNo ratings yet
- Sample 7613Document11 pagesSample 7613VikashKumarNo ratings yet
- WINSEM2019-20 MEE1002 TH VL2019205001913 DA-1 QP KEY Assignment IDocument9 pagesWINSEM2019-20 MEE1002 TH VL2019205001913 DA-1 QP KEY Assignment IDebdoot GhoshNo ratings yet
- Tests For Gas Permeability of ConcreteDocument6 pagesTests For Gas Permeability of ConcreteAzuriak1No ratings yet
- LNG Receiving Terminals: BY CH - Satvika 16021A2545Document24 pagesLNG Receiving Terminals: BY CH - Satvika 16021A2545Ram Charan Konidela100% (1)
- Shadan Zolghani - 9 Ag Displacement and VelocityDocument12 pagesShadan Zolghani - 9 Ag Displacement and Velocityapi-531290004No ratings yet
- Compact Heat Exchanger DesignDocument52 pagesCompact Heat Exchanger DesignoperationmanagerNo ratings yet
- ELT1130 Anatomy of A Robot NotesDocument15 pagesELT1130 Anatomy of A Robot NotesEd PawliwNo ratings yet
- Material Balance: Lecture By: Ir. Dewi Tristantini Mt. Phd. University of IndonesiaDocument29 pagesMaterial Balance: Lecture By: Ir. Dewi Tristantini Mt. Phd. University of IndonesiaEdward Gustaf100% (3)
- Introduction To Quaternions With Numerou PDFDocument249 pagesIntroduction To Quaternions With Numerou PDFRicardoNo ratings yet
- 2 Principles of Roof Truss DesignDocument10 pages2 Principles of Roof Truss Designabhi aroteNo ratings yet
- Cohesive Energy 1Document5 pagesCohesive Energy 1kalloliNo ratings yet
- 1 - Pdfsam - 51 - Pdfsam - Jane Bennett Vibrant Matter A Political Ecology of Things 2010 PDFDocument25 pages1 - Pdfsam - 51 - Pdfsam - Jane Bennett Vibrant Matter A Political Ecology of Things 2010 PDFAnna PrzytomskaNo ratings yet
- PhysicsDocument28 pagesPhysicsamritam yadavNo ratings yet
- Yang Et Al. (2017)Document11 pagesYang Et Al. (2017)Francisco OppsNo ratings yet
- t9 PDFDocument21 pagest9 PDFselvaganapathy1992No ratings yet
- Modified Moment Estimation For A Two Parameter Gamma DistributionDocument9 pagesModified Moment Estimation For A Two Parameter Gamma DistributionInternational Organization of Scientific Research (IOSR)No ratings yet
- Nonlinear Analysis Methods For Reinforced Concrete Buildings With Shear WallsDocument8 pagesNonlinear Analysis Methods For Reinforced Concrete Buildings With Shear Wallsakif-benzer-6764No ratings yet
- Welding of 4140 & 316Document5 pagesWelding of 4140 & 316Engineer AnasNo ratings yet
- Frida Kunti Setiowati, Barlah Rumhayati: Seminar Nasional XI Pendidikan Biologi FKIP UNS 125Document6 pagesFrida Kunti Setiowati, Barlah Rumhayati: Seminar Nasional XI Pendidikan Biologi FKIP UNS 125Ridwan PutraNo ratings yet
- FredHymans TheoryRopeTraction Part1Document11 pagesFredHymans TheoryRopeTraction Part1WojciechNo ratings yet
- HAZOP Work Sheet ZaltoprofenDocument26 pagesHAZOP Work Sheet Zaltoprofenkirandevi1981No ratings yet
- 2017 - OPUS Quant Advanced PDFDocument205 pages2017 - OPUS Quant Advanced PDFIngeniero Alfonzo Díaz Guzmán100% (1)
- Flexibility FactorsDocument61 pagesFlexibility FactorsCarlos BorgesNo ratings yet
- Class XI Half SlybussDocument10 pagesClass XI Half SlybussDevansh AgarwalNo ratings yet
- (NagpurStudents - Org) Advanced PhysicsDocument4 pages(NagpurStudents - Org) Advanced PhysicsVaibhav NardangeNo ratings yet
- TB PhysicsTestY3 654b29bcc38e07.654b29bed4Document17 pagesTB PhysicsTestY3 654b29bcc38e07.654b29bed4Prakash ManchukondaNo ratings yet
- General Contractor, Supplier, Independent Survey, Ship Service & IndustrialDocument24 pagesGeneral Contractor, Supplier, Independent Survey, Ship Service & IndustrialNicoNo ratings yet
- How To Design Roof Purlins - A Solved Example - StructvilleDocument16 pagesHow To Design Roof Purlins - A Solved Example - StructvilleLavanyanNo ratings yet
- Modeling and Optimization of An Auto-Thermal Ammonia Synthesis Reactor Using The Gravitational Search AlgorithmDocument8 pagesModeling and Optimization of An Auto-Thermal Ammonia Synthesis Reactor Using The Gravitational Search AlgorithmJen ChavezNo ratings yet