Professional Documents
Culture Documents
Whole Cell Extract
Uploaded by
biotech_vidhyaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Whole Cell Extract
Uploaded by
biotech_vidhyaCopyright:
Available Formats
WHOLE CELL EXTRACT PREPARATION
Materials:
plate of cells (80-100% confluent)
cold 1xPBS
cell scraper (Midwest Scientific #TP9902)
dry ice/methanol
Buffer C
Bio-Rad Protein Assay Dye Reagent Concentrate (cat#500-0006)
Procedure:
1. Aspirate off media from 10 cm TC plate.
2. Rinse with cold 1xPBS (pipet about 2ml onto plate and swirl around gently).
Aspirate off 1xPBS.
3. Add 1 ml cold 1xPBS to plate.
4. Scrape cells to one area (at the edge of the plate).
5. Pipet cells into microfuge tube.
6. Spin cells for 10 seconds in microfuge tube.
7. Aspirate off 1xPBS (be careful not to lose the pellet).
8. Freeze cell pellet in dry ice/methanol.
9. Resuspend pellet in 20µl Buffer C.
Buffer C (100ml) Stock Volume
25% glycerol 100% 25 ml
0.42 M NaCl 4M 10.5 ml
1.5 mM MgCl2 1M 150 µl
0.2 mM EDTA 0.5 M 40 µl
20 mM HEPES (pH 7.9) 1M 2 mls
• add distilled water up to 100 ml
• add DTT and PMSF to 0.5 mM before use
10. Spin tube for 15 seconds.
11. The proteins are suspended in the supernatant. Collect the supernatant to
determine the protein concentration by BioRad assay.
Submitted by: Sue Fox
III.C.3
You might also like
- Plant and Animal Bio-Chemistry - Including Information on Amino Acids, Proteins, Pigments and Other Chemical Constituents of Organic MatterFrom EverandPlant and Animal Bio-Chemistry - Including Information on Amino Acids, Proteins, Pigments and Other Chemical Constituents of Organic MatterNo ratings yet
- Cell starvation comet assayDocument5 pagesCell starvation comet assayShuying WuNo ratings yet
- Yeast SmashDocument5 pagesYeast Smashme_dayakarNo ratings yet
- The Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterFrom EverandThe Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterNo ratings yet
- Column BufferDocument2 pagesColumn BufferRana Mazhar AbbasNo ratings yet
- 100 Recipes of French Cooking for Christmas and HolidaysFrom Everand100 Recipes of French Cooking for Christmas and HolidaysNo ratings yet
- Genetic Engineering Lab ManualDocument11 pagesGenetic Engineering Lab ManualGeetanjali GorainNo ratings yet
- Standard methods for the examination of water and sewageFrom EverandStandard methods for the examination of water and sewageNo ratings yet
- Western Blot Protocol SummaryDocument12 pagesWestern Blot Protocol Summarysms143No ratings yet
- Helpful Edman Degradation Sample Preparation ProtocolsDocument7 pagesHelpful Edman Degradation Sample Preparation ProtocolsThis guyNo ratings yet
- Isolation of Neonatal Rat Myocytes: ADS BufferDocument4 pagesIsolation of Neonatal Rat Myocytes: ADS BufferMohammad AliNo ratings yet
- Extraction of DNA From BacteriaDocument6 pagesExtraction of DNA From BacteriaMeetali GuptaNo ratings yet
- DNA Extraction From Fungi, Yeast, and BacteriaDocument2 pagesDNA Extraction From Fungi, Yeast, and Bacteriavishankgupta100% (1)
- Isolasi Dna: Dna and Rna Extractions Dna and Rna ExtractionsDocument5 pagesIsolasi Dna: Dna and Rna Extractions Dna and Rna ExtractionsPspduntanDuaribusebelasNo ratings yet
- STE Buffer ProtocolDocument3 pagesSTE Buffer ProtocolJasminSutkovicNo ratings yet
- Advanced Preparation ADNDocument2 pagesAdvanced Preparation ADNJorgeAngeliniNo ratings yet
- CTAB DNA ExtractionDocument2 pagesCTAB DNA ExtractionHumam-bmNo ratings yet
- Preparation of Taq DNA PolymeraseDocument3 pagesPreparation of Taq DNA PolymeraseGerardo David Gonzalez EstradaNo ratings yet
- Isolation of Plant Genomic DNA (Draft - 2)Document4 pagesIsolation of Plant Genomic DNA (Draft - 2)Prayash NayakNo ratings yet
- Competent Cells R BCLDocument2 pagesCompetent Cells R BCLme_dayakarNo ratings yet
- Dna07 2Document2 pagesDna07 2Sabesan TNo ratings yet
- DNA ExtractionDocument73 pagesDNA ExtractionMustafa KhandgawiNo ratings yet
- Western Immunoblotting ProtocolDocument8 pagesWestern Immunoblotting ProtocolJKayckeNo ratings yet
- Protein Prep - ScribdDocument2 pagesProtein Prep - ScribdEvan Andrew LangilleNo ratings yet
- 2-D Polyacrylamide Gel ElectrophoresisDocument9 pages2-D Polyacrylamide Gel Electrophoresisbiosa45100% (2)
- Lang Lab TEV Protease ProtocolDocument2 pagesLang Lab TEV Protease ProtocolEvan Andrew LangilleNo ratings yet
- Extraction of DNA From Whole BloodDocument5 pagesExtraction of DNA From Whole BloodvishankguptaNo ratings yet
- General Protocol For Western BlottingDocument2 pagesGeneral Protocol For Western BlottingMazeNo ratings yet
- Bulletin 6376 PDFDocument2 pagesBulletin 6376 PDFSky ImranNo ratings yet
- GST protein purification optimizationDocument5 pagesGST protein purification optimizationRay KuoNo ratings yet
- Nib Dna Extraction ProtocolDocument1 pageNib Dna Extraction Protocolpreethi.elizabeth2022No ratings yet
- Tail Genomic DNADocument1 pageTail Genomic DNATatiana H. GomezNo ratings yet
- Protein Precipitation Protocols PDFDocument6 pagesProtein Precipitation Protocols PDFJeTiKNo ratings yet
- Metaphase Preparation From Adherent CellsDocument2 pagesMetaphase Preparation From Adherent CellsDaniela Mădălina GhețuNo ratings yet
- Telomeric PNA FISH On Metaphase Chromosomes: Required Solutions/Reagents Blocking Reagent (Roche 11096176001)Document3 pagesTelomeric PNA FISH On Metaphase Chromosomes: Required Solutions/Reagents Blocking Reagent (Roche 11096176001)biosynthesis12No ratings yet
- Protocolo de Extracción de ADN Bacteriano - AusubelDocument5 pagesProtocolo de Extracción de ADN Bacteriano - AusubelLesly CastilloNo ratings yet
- Transformation of YeastDocument2 pagesTransformation of YeastandrascellbiolNo ratings yet
- Isolation of Plasmid Dna From Escherichia Coli: by STET (Rapid) MethodDocument4 pagesIsolation of Plasmid Dna From Escherichia Coli: by STET (Rapid) MethodVijayasarathy Sampath KumarNo ratings yet
- LAB Manual 1Document2 pagesLAB Manual 1syazaismailNo ratings yet
- ProtocolDocument16 pagesProtocolTran Tu NguyenNo ratings yet
- Isolation of Plant Genomic DNADocument10 pagesIsolation of Plant Genomic DNAChris PenielNo ratings yet
- 1a. Alkaline Lysis MethodDocument2 pages1a. Alkaline Lysis MethodParijat BanerjeeNo ratings yet
- Practicals MSC Mol Biol 8-10 PDFDocument8 pagesPracticals MSC Mol Biol 8-10 PDFAnupamNo ratings yet
- Bacterial DNA IsolationDocument6 pagesBacterial DNA IsolationVikram SinghNo ratings yet
- Yeast ChIP Protocol: Mechanical Breakage & FA Lysis BufferDocument8 pagesYeast ChIP Protocol: Mechanical Breakage & FA Lysis BuffersurendrasrawanNo ratings yet
- DNA Isolation From Spleen ProtocolDocument2 pagesDNA Isolation From Spleen ProtocolSherlock Wesley ConanNo ratings yet
- Isolation of Genomic DNA from MycobacteriaDocument2 pagesIsolation of Genomic DNA from MycobacteriaGuhan KANo ratings yet
- DNA Prep from BloodDocument3 pagesDNA Prep from BloodGAURAVNo ratings yet
- Homemade Plasmid Mini PrepDocument4 pagesHomemade Plasmid Mini PrepBada HanNo ratings yet
- Nuclear ExtractsDocument2 pagesNuclear Extractsbiotech_vidhyaNo ratings yet
- General Molecular Biology Laboratory ProtocolsDocument4 pagesGeneral Molecular Biology Laboratory ProtocolsDan MontagnaNo ratings yet
- Plant Genomic Dna Extraction by Ctab 2 FionaDocument3 pagesPlant Genomic Dna Extraction by Ctab 2 FionaMB avonpclk.comNo ratings yet
- General Immunohistochemistry ProtocolDocument3 pagesGeneral Immunohistochemistry Protocolpooja_futureNo ratings yet
- Plant Genomic DNA Extraction by CTAB - 2 - FionaDocument5 pagesPlant Genomic DNA Extraction by CTAB - 2 - FionayomnayasminNo ratings yet
- Western Blot Protocol for GPCR AntibodiesDocument3 pagesWestern Blot Protocol for GPCR Antibodiessathakoil7501No ratings yet
- DNA Extraction SOPDocument15 pagesDNA Extraction SOPattiyaNo ratings yet
- Isolated Cardiac MitochondriaDocument7 pagesIsolated Cardiac MitochondriaCristina Espadas ÑíguezNo ratings yet
- PCI Extraction PDFDocument3 pagesPCI Extraction PDFHanifHarySNo ratings yet
- 4) DNA ExtractionDocument11 pages4) DNA ExtractionajiesyahbarieNo ratings yet
- SDS PageDocument2 pagesSDS Pagebiotech_vidhyaNo ratings yet
- BT 2019Document13 pagesBT 2019biotech_vidhyaNo ratings yet
- Facs ProtocolDocument7 pagesFacs ProtocolmisterxNo ratings yet
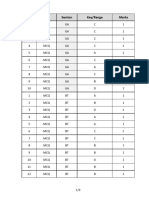
- Q.No. Type Section Key/Range MarksDocument3 pagesQ.No. Type Section Key/Range Marksbiotech_vidhyaNo ratings yet
- Stripping For ReprobingDocument2 pagesStripping For ReprobingStella SalvatoreNo ratings yet
- Components Reaction MixtureDocument3 pagesComponents Reaction Mixturebiotech_vidhyaNo ratings yet
- Stripping For ReprobingDocument2 pagesStripping For ReprobingStella SalvatoreNo ratings yet
- Troubleshooting SDS-PAGE 1Document3 pagesTroubleshooting SDS-PAGE 1biotech_vidhyaNo ratings yet
- Brad FordDocument12 pagesBrad FordQi ChaoNo ratings yet
- TNPSC Group 1 Prelim Book List PDFDocument2 pagesTNPSC Group 1 Prelim Book List PDFbiotech_vidhyaNo ratings yet
- Polymerasen GuideDocument16 pagesPolymerasen Guidebiotech_vidhyaNo ratings yet
- Polymerase Chain Reaction (PCR)Document3 pagesPolymerase Chain Reaction (PCR)biotech_vidhyaNo ratings yet
- Buffer Preparation Guide for DNA/Protein Work (Shi LabDocument6 pagesBuffer Preparation Guide for DNA/Protein Work (Shi Labbiotech_vidhyaNo ratings yet
- Buffer Preparation Guide for DNA/Protein Work (Shi LabDocument6 pagesBuffer Preparation Guide for DNA/Protein Work (Shi Labbiotech_vidhyaNo ratings yet
- TNPSC Group 1 Prelim Book List PDFDocument2 pagesTNPSC Group 1 Prelim Book List PDFbiotech_vidhyaNo ratings yet
- Nuclear ExtractsDocument2 pagesNuclear Extractsbiotech_vidhyaNo ratings yet
- TNPSC Group 1 Prelim Book List PDFDocument2 pagesTNPSC Group 1 Prelim Book List PDFbiotech_vidhyaNo ratings yet
- Befcv List PDFDocument22 pagesBefcv List PDFbiotech_vidhyaNo ratings yet
- Ies 17 Set A Me Q ADocument67 pagesIes 17 Set A Me Q Abiotech_vidhyaNo ratings yet
- Img Word-To PDFDocument3 pagesImg Word-To PDFbiotech_vidhyaNo ratings yet
- Qpaper PondyDocument21 pagesQpaper Pondybiotech_vidhyaNo ratings yet
- ESE 2017 Mechanical Engineering Prelims Exam Detailed SolutionDocument52 pagesESE 2017 Mechanical Engineering Prelims Exam Detailed SolutionpataNo ratings yet
- A.E. (Mechanical Engineering I) 2007Document24 pagesA.E. (Mechanical Engineering I) 2007Mukesh KumarNo ratings yet
- TDC 41597 A (Mechanical Engg.) - 2012Document20 pagesTDC 41597 A (Mechanical Engg.) - 2012biotech_vidhyaNo ratings yet
- Qpaper PondyDocument21 pagesQpaper Pondybiotech_vidhyaNo ratings yet
- Mechanical Engineering Code No. 14: Combined Competitive (Preliminary) Examination, 2010Document20 pagesMechanical Engineering Code No. 14: Combined Competitive (Preliminary) Examination, 2010biotech_vidhyaNo ratings yet
- Recruitment RulesDocument5 pagesRecruitment Rulesbiotech_vidhyaNo ratings yet
- 1 TolerancesDocument1 page1 Tolerancesbiotech_vidhyaNo ratings yet
- Part and Mold Design GuideDocument170 pagesPart and Mold Design GuideminhtintinNo ratings yet
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 3.5 out of 5 stars3.5/5 (2)
- The Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionFrom EverandThe Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionRating: 4 out of 5 stars4/5 (811)
- 10% Human: How Your Body's Microbes Hold the Key to Health and HappinessFrom Everand10% Human: How Your Body's Microbes Hold the Key to Health and HappinessRating: 4 out of 5 stars4/5 (33)
- All That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesFrom EverandAll That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesRating: 4.5 out of 5 stars4.5/5 (397)
- Human: The Science Behind What Makes Your Brain UniqueFrom EverandHuman: The Science Behind What Makes Your Brain UniqueRating: 3.5 out of 5 stars3.5/5 (38)
- Tales from Both Sides of the Brain: A Life in NeuroscienceFrom EverandTales from Both Sides of the Brain: A Life in NeuroscienceRating: 3 out of 5 stars3/5 (18)
- Crypt: Life, Death and Disease in the Middle Ages and BeyondFrom EverandCrypt: Life, Death and Disease in the Middle Ages and BeyondRating: 4 out of 5 stars4/5 (3)
- Who's in Charge?: Free Will and the Science of the BrainFrom EverandWho's in Charge?: Free Will and the Science of the BrainRating: 4 out of 5 stars4/5 (65)
- Good Without God: What a Billion Nonreligious People Do BelieveFrom EverandGood Without God: What a Billion Nonreligious People Do BelieveRating: 4 out of 5 stars4/5 (66)
- The Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindFrom EverandThe Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindRating: 4.5 out of 5 stars4.5/5 (93)
- This Is Your Brain On Parasites: How Tiny Creatures Manipulate Our Behavior and Shape SocietyFrom EverandThis Is Your Brain On Parasites: How Tiny Creatures Manipulate Our Behavior and Shape SocietyRating: 3.5 out of 5 stars3.5/5 (31)
- Undeniable: How Biology Confirms Our Intuition That Life Is DesignedFrom EverandUndeniable: How Biology Confirms Our Intuition That Life Is DesignedRating: 4 out of 5 stars4/5 (11)
- The Mind & The Brain: Neuroplasticity and the Power of Mental ForceFrom EverandThe Mind & The Brain: Neuroplasticity and the Power of Mental ForceNo ratings yet
- The Lives of Bees: The Untold Story of the Honey Bee in the WildFrom EverandThe Lives of Bees: The Untold Story of the Honey Bee in the WildRating: 4.5 out of 5 stars4.5/5 (44)
- The Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceFrom EverandThe Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceRating: 4.5 out of 5 stars4.5/5 (515)
- The Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorFrom EverandThe Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorNo ratings yet
- Eels: An Exploration, from New Zealand to the Sargasso, of the World's Most Mysterious FishFrom EverandEels: An Exploration, from New Zealand to the Sargasso, of the World's Most Mysterious FishRating: 4 out of 5 stars4/5 (30)
- A Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsFrom EverandA Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsRating: 4.5 out of 5 stars4.5/5 (4)
- Wayfinding: The Science and Mystery of How Humans Navigate the WorldFrom EverandWayfinding: The Science and Mystery of How Humans Navigate the WorldRating: 4.5 out of 5 stars4.5/5 (18)
- The Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and IntestineFrom EverandThe Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and IntestineRating: 4 out of 5 stars4/5 (17)
- Darwin's Dangerous Idea: Evolution and the Meaning of LifeFrom EverandDarwin's Dangerous Idea: Evolution and the Meaning of LifeRating: 4 out of 5 stars4/5 (523)
- Why We Sleep: Unlocking the Power of Sleep and DreamsFrom EverandWhy We Sleep: Unlocking the Power of Sleep and DreamsRating: 4.5 out of 5 stars4.5/5 (2083)
- Lymph & Longevity: The Untapped Secret to HealthFrom EverandLymph & Longevity: The Untapped Secret to HealthRating: 4.5 out of 5 stars4.5/5 (13)
- Buddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomFrom EverandBuddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomRating: 4 out of 5 stars4/5 (215)