Professional Documents
Culture Documents
Binary Distillation: Experiment 1
Binary Distillation: Experiment 1
Uploaded by
Jocelyn CorpuzOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Binary Distillation: Experiment 1
Binary Distillation: Experiment 1
Uploaded by
Jocelyn CorpuzCopyright:
Available Formats
Experiments in Chemical Engineering Binary Distillation
Experiment 1
BINARY DISTILLATION
INTRODUCTION
Distillation is one of the most important but also the most energy intensive separation
process that chemical engineers should be concerned about. Thus, a good understanding of the
theories and application of this unit operation is essential in order to minimize energy
consumption. There are many types of distillation operations, depending on the process
followed, the type of equipment and the number of volatile components present in the solution
to be purified. In this laboratory exercise the one using the concept of a binary equilibrium
relationship by operating a simple batch distillation set-up is investigated. The binary system
applied is ethanol-water where students will investigate the validity of the Rayleigh’s equation.
OBJECTIVES
The experiment is composed of two parts with the following objectives:
1. To investigate the applicability of the Rayleigh’s Equation to describe differential
distillation.
2. To determine the temperature and concentration profile in a differential distillation
process.
Unit Operations Laboratory Page 1
Experiments in Chemical Engineering Binary Distillation
THEORY
Distillation is a unit operation commonly used for purifying liquids and separating a
liquid mixture of miscible and volatile substances into individual components or, in some cases,
into groups of components. A liquid can be classified as volatile when it is readily vaporized at a
relatively low temperature. The boiling of the more volatile components of the mixture drives
the distillation process. When the vapor is cooled, the more volatile material condenses in a
greater proportion than the less volatile material.
The components are separated based on their physical properties, specifically, relative
volatilities. Relative volatility, a tool used to express the magnitude of the equilibrium
distribution, is defined as
(𝑦𝐴 ⁄𝑥𝐴 )
𝛼=
(𝑦𝐵 ⁄𝑥𝐵 )
where 𝛼 = Relative volatility
𝑦 = Vapor composition
𝑥 = Liquid composition
𝐴 = More volatile component
𝐵 = Less volatile component
There are two types of distillation classified according to its mode of operation. These
are continuous and the batch distillation. In a batch system, the column can handle different
mixtures by simply changing its operating conditions. The main disadvantage to using this kind
of system, however, is that the longer the components are exposed to high temperature, the
better the chances that the components are broken down via thermal degradation. Along with
this, the energy requirements are usually higher for a batch system. Conversely, continuous
distillation, as the name implies, feed is continuously supplied and separated into two or more
products over some period. On the other hand, batch distillation involves the processing of a
batch of feed at a given time and split into products by selectively removing the more volatile
fractions over time.
A. Differential Distillation
When a binary solution of volatile liquids is subjected to batch differential distillation,
the concentration of the solution in the distillery still decreases in the more volatile component
as the boiling point of the solution increases. The resulting vapor is allowed to condense
through a condenser and collected together as the distillate product.
Unit Operations Laboratory Page 2
Experiments in Chemical Engineering Binary Distillation
The problem in this case is to relate the initial amount of feed together with its concentration
and the amount of over-all distillate collected and its concentration.
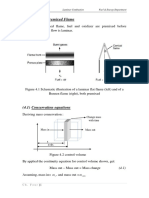
At any given time, the concentrations may be related by material balance. Referring to
Figure 1, if W is the amount in the still at any time of concentration x, a more volatile
component balance yields
X
𝑤𝑥 − (𝑤 − 𝑑𝑊)(𝑥 − 𝑑𝑥) = 𝑦𝑑𝑊
𝑤𝑥 − 𝑤𝑥 + 𝑥𝑑𝑊 + 𝑊𝑑𝑥 − 𝑑𝑊𝑑𝑥 = 𝑦𝑑𝑊
𝑊𝑑𝑥 = (𝑦 − 𝑥)𝑑𝑊
y
𝑑𝑊 𝑑𝑥
= dW
𝑊 𝑦−𝑥
x W-dW
Figure 1: Distillation System
Integrating, considering the initial feed as F and concentration xF,
𝑊 𝑥𝑤
𝑑𝑊 𝑑𝑥
∫ =∫
𝐹 𝑊 𝑥𝐹 𝑦 − 𝑥
𝑥𝐹
𝐹 𝑑𝑥
𝑙𝑛 = ∫
𝑊 𝑦−𝑥
𝑥𝑤
This equation is referred to as Rayleigh’s Equation. The instantaneous liquid
composition, x, and the corresponding vapor composition, y are assumed to be in equilibrium
with one another. Vapor Liquid Equilibria (VLE) data may be determined from Vapor-Pressure-
Temperature, Boiling Point Composition diagram or equilibrium data, or x-y diagram for the
binary system.
Unit Operations Laboratory Page 3
Experiments in Chemical Engineering Binary Distillation
The Rayleigh Equation may be simplified to various forms such as:
1. If Henry’s law applies, y = mx where m = H/P. The integrated equation yields
𝐹 1 𝑥𝐹
𝑙𝑛 = 𝑙𝑛
𝑊 𝑚 𝑥𝑊
this equation, however, is applicable only for dilute solutions, where the partial pressure of the
vapor is a linear function of composition.
2. If the relative volatility, 𝛼AB may be assumed practically constant then,
𝛼𝐴𝐵
𝑦=
(𝛼𝐴𝐵 − 1)𝑥 + 1
substituting this in the previous equation and integrating gives
𝐹 1 𝑥𝐹 1 − 𝑥𝑤
𝑙𝑛 = [𝑙𝑛 +̅̅̅̅̅̅
𝛼𝐴𝐵 𝑙𝑛 ]
𝑊 𝛼 ̅̅̅̅̅
𝐴𝐵 − 1 𝑥𝑤 1 − 𝑥𝐹
or in terms of individual components,
𝐴1 𝐵1
𝑙𝑛 = ̅̅̅̅̅
𝛼𝐴𝐵 𝑙𝑛
𝐴2 𝐵2
Subscript 1 refers to the initial amounts and subscript 2 refers to the final amounts
found in the solution. The amount of distillate may be determined by material balance together
with its composition.
If the above assumptions could not be applied, the best way to evaluate the integral of
Equation 2 is by graphical or numerical methods. The graphical method is shown in Figure 2.
1
Graphically, plot 𝑦−𝑥 versus x at different stages of the experiment to relate the amount
distilled at any time with composition and temperature by getting the area under the curve and
comparing this area and composition with the experimental data obtained. The validity of the
simplified equation may also be compare with the experimental data.
𝐹
𝐴𝑟𝑒𝑎 = 𝑙𝑛 𝑊
1
𝑦−𝑥
𝑥𝑤 𝑥 𝑥𝐹
Figure 2: Graphical Method
Unit Operations Laboratory Page 4
Experiments in Chemical Engineering Binary Distillation
EQUIPMENT
A. Actual Equipment
Control System
Packed Column
Boiler Still
Figure 3: Laboratory Distillation Column
B. Equipment Design
The equipment contains a 4-inch bubble cap batch distillation column which may be
operated under total or partial reflux conditions. Also used in this experiment is a
distillation column packed with glass beads for total reflux operations. As for the
determination of the refractive index, a refractometer was used.
Unit Operations Laboratory Page 5
Experiments in Chemical Engineering Binary Distillation
PROCEDURE
A. Preparation of a Calibration Curve
1. Prepare a 0, 10, 20, 30, 40, 50, 60, 70, 80, 90 (% v/v) ethanol solution.
2. Use the refractometer to determine the refractive indices of the different
solutions.
3. Plot the refractive index versus ethanol concentration.
B. Batch or Differential Distillation Experiment
1. Setup the batch distillation apparatus.
2. Make 10% ethanol (25 mL pure ethanol and 225 mL water).
3. Heat the setup until the first drop of condensate is collected. Record the initial
temperature reading and start the time.
4. For every 10 mL of condensate collected, record the corresponding time and
temperature.
5. Using a refractometer, determine the refractive index of the samples collected.
6. Repeat steps 4 and 5 until you have at least six runs or until 60 to 70% of the
solution is distilled.
Unit Operations Laboratory Page 6
Experiments in Chemical Engineering Binary Distillation
Binary Distillation
DATA SHEET
A. Calibration Data
Concentration of Concentration of
C2H5OH-H2O Refractive Index C2H5OH-H2O Refractive Index
(% v/v) (% v/v)
0 1.3325 60 1.3595
10 1.336 70 1.361
20 1.315 80 1.361
30 1.354 90 1.360
40 1.3555 99 1.3595
50 1.360
B. Batch Distillation
Condensate Bottom Sample Middle Sample
Volume Time
(mL) Temperature Refractive Temperature Refractive Temperature Refractive (min’sec’’)
(°C) Index (°C) Index (°C) Index
1st drop 45 0 45 0 45 0 -----
10 88 1.361 75 1.34 65 1.3615 1’08’’
20 88 1.3615 78 1.339 75 1.361 2’18’’
30 89 1.36155 83 1.3385 79 1.3605 3’30’’
40 90.5 1.362 85 1.338 83 1.36 4’49’’
50 92 1.3605 87 1.336 87 1.3585 6’14’’
60 94 1.36 89 1.3355 89 1.358 7’49’’
70 95 1.3585 91 1.334 92 1.355 9’37’’
80 96 1.354 93 1.3333 94.5 1.35 11’39’’
90 98 1.348 94 1.3333 96.5 1.345 13’58’’
100 98 1.339 95 1.3326 98 1.3385 16’24’’
Unit Operations Laboratory Page 7
Experiments in Chemical Engineering Binary Distillation
GUIDE QUESTIONS
1. Plot the calibration curve as refractive index versus ethanol concentration.
2. Derive a correlation equation for refractive index as a function of concentration.
Comment on the usefulness of this correlation. What is the range of concentration that
generates the smallest and largest deviation?
3. Plot the F/W and the experimental ratio F/W versus the concentration of the residue.
Describe the results. Is there good agreement between the experimental results with
those obtained by Rayleigh’s equation?
4. Compare the experimental amount of total collected with the theoretical results using
the graphical method of solution and using the average relative volatility during the
entire differential distillation process. Tabulate and indicate the percent deviation.
ANALYSES AND CALCULATIONS
Unit Operations Laboratory Page 8
Experiments in Chemical Engineering Binary Distillation
APPENDIX
Unit Operations Laboratory Page 9
You might also like
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationFrom EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNo ratings yet
- Exp - 2 Bubble Cap Distillation ColumnDocument13 pagesExp - 2 Bubble Cap Distillation ColumnAdawiyah Al-jufri100% (1)
- Batch Reactive DistillationDocument7 pagesBatch Reactive DistillationChalmer BelaroNo ratings yet
- Vapor Liquid EquilibriumDocument28 pagesVapor Liquid EquilibriumKhloud MadihNo ratings yet
- Mass Transfer 16CE2004 - HandoutDocument80 pagesMass Transfer 16CE2004 - HandoutjAYNo ratings yet
- Minimum Reflux RatioDocument9 pagesMinimum Reflux RatioEmmanuelNo ratings yet
- Assignment Thermal UiTMDocument29 pagesAssignment Thermal UiTMiwe1234No ratings yet
- Practice Problems in Absorption and HumidificationDocument4 pagesPractice Problems in Absorption and HumidificationJenna BraszNo ratings yet
- DistillationDocument124 pagesDistillationasharab70100% (1)
- Counter-Current Extraction: An Introduction to the Design and Operation of Counter-Current ExtractorsFrom EverandCounter-Current Extraction: An Introduction to the Design and Operation of Counter-Current ExtractorsNo ratings yet
- PP-309 Mass Transfer: Course Facilitator: Nadia Khan Lecture of Week 1 & 2Document77 pagesPP-309 Mass Transfer: Course Facilitator: Nadia Khan Lecture of Week 1 & 2ashas waseem100% (1)
- Liquid Liquid Extraction in A Packed BedDocument26 pagesLiquid Liquid Extraction in A Packed BedAr SaidNo ratings yet
- Heat-Mass Transfer in A Tubular Chemical Reactor: Rehena NasrinDocument11 pagesHeat-Mass Transfer in A Tubular Chemical Reactor: Rehena NasrinNaveed KhanNo ratings yet
- Topic 3.2 - Internal Diffusion and ReactionDocument36 pagesTopic 3.2 - Internal Diffusion and ReactionHamdan Azman100% (1)
- CSTR 40LDocument16 pagesCSTR 40LhishamNo ratings yet
- Thermodynamic Properties, Equations of State, Methods Used To Describe and Predict Phase EquilibriaDocument20 pagesThermodynamic Properties, Equations of State, Methods Used To Describe and Predict Phase EquilibriaanisfathimaNo ratings yet
- Mccabe ThieleDocument63 pagesMccabe ThieleAshlesh MangrulkarNo ratings yet
- 2 Lewis Sorel MethodDocument18 pages2 Lewis Sorel MethodStanley SibandaNo ratings yet
- On Off Level Control ProcessDocument16 pagesOn Off Level Control ProcessAndrew NabilNo ratings yet
- 3 - Vle in A Binary Mixture - 2Document22 pages3 - Vle in A Binary Mixture - 2Faisal El HakimNo ratings yet
- Chapter 1 - Part IDocument46 pagesChapter 1 - Part IMaisarah RazaliNo ratings yet
- Cre 1 IntroductionDocument4 pagesCre 1 IntroductionEvangeline LauNo ratings yet
- CHE504 - Lab Report On Distillation ColuDocument27 pagesCHE504 - Lab Report On Distillation ColuMuhammad Irfan MalikNo ratings yet
- P4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperatureDocument7 pagesP4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperaturejayaprinaNo ratings yet
- Henrys Law Solved ProblemsDocument3 pagesHenrys Law Solved ProblemsayushNo ratings yet
- Separation Process Engineering CHEN 312: Ys18@aub - Edu.lbDocument28 pagesSeparation Process Engineering CHEN 312: Ys18@aub - Edu.lbsoe0303No ratings yet
- Final Report PFRDocument12 pagesFinal Report PFRmark_ancotNo ratings yet
- CHE 312 Lecture 2Document17 pagesCHE 312 Lecture 2Muhammad Hamza EjazNo ratings yet
- C4 Lab ReportDocument11 pagesC4 Lab ReportchaitanyaNo ratings yet
- Vapor Liquid EquilibriumDocument25 pagesVapor Liquid EquilibriumHariKrishnaBushi100% (1)
- 01 Lecture Note - Binary Flash DistillationDocument21 pages01 Lecture Note - Binary Flash DistillationtimNo ratings yet
- Al Duri Tutorial1 AbsorptionDocument2 pagesAl Duri Tutorial1 AbsorptionJia YiNo ratings yet
- 7 1. Vapor Liquid EquilibriumDocument9 pages7 1. Vapor Liquid Equilibriumwaseemkhan49No ratings yet
- Sample Problems On Gas AbsorptionDocument2 pagesSample Problems On Gas AbsorptionKevin Laganao67% (3)
- Characterization of Solid ParticlesDocument8 pagesCharacterization of Solid ParticlesShary Mosquera50% (2)
- LESSON 8 - Shell Momentum BalanceDocument66 pagesLESSON 8 - Shell Momentum BalancePamela MendozaNo ratings yet
- CSTRDocument20 pagesCSTRSharing Caring100% (1)
- Phase EquilibriumDocument32 pagesPhase EquilibriumRaihan PradanaNo ratings yet
- Lab6-Tubular Flow ReactorDocument11 pagesLab6-Tubular Flow ReactorNurtasha Atikah100% (1)
- Mass Transfer - AbsorptionDocument39 pagesMass Transfer - AbsorptionnivedhithaNo ratings yet
- (4.1) Laminar Premixed FlameDocument31 pages(4.1) Laminar Premixed Flameمصطفى العباديNo ratings yet
- Distillation TutorialDocument17 pagesDistillation TutorialXin-YiWoon100% (1)
- VLE Lab Report 2015ssdaDocument37 pagesVLE Lab Report 2015ssdaRafiHunJian0% (1)
- Chapter 4Document43 pagesChapter 4aliNo ratings yet
- CHAPTER 2 Single Particle in A Fluid - 724666540Document6 pagesCHAPTER 2 Single Particle in A Fluid - 724666540Donna Mae Ramos GalaezNo ratings yet
- Week 4 - Vapor-Liquid Separation (Multicomponent Distillation)Document19 pagesWeek 4 - Vapor-Liquid Separation (Multicomponent Distillation)psychopassNo ratings yet
- SI Heat 5e Chap02 LectureDocument84 pagesSI Heat 5e Chap02 Lecturepremnath chakriNo ratings yet
- Thermal Laboratory - Lab Experiment-1Document7 pagesThermal Laboratory - Lab Experiment-1Raj PratyushNo ratings yet
- Single Effect Evaporator 2Document22 pagesSingle Effect Evaporator 2Shailesh Lohare100% (1)
- Sep Lab Exp 1 LatestDocument20 pagesSep Lab Exp 1 LatestChan Chun ChenNo ratings yet
- CONTINUOUS DistillationDocument5 pagesCONTINUOUS DistillationNaseer SattarNo ratings yet
- Packed Absorption and Stripping Columns: Prof. Dr. Marco Mazzotti - Institut Für VerfahrenstechnikDocument14 pagesPacked Absorption and Stripping Columns: Prof. Dr. Marco Mazzotti - Institut Für Verfahrenstechnikishhh12No ratings yet
- Ergun Equation ValidationDocument18 pagesErgun Equation ValidationAtikur Rahman100% (1)
- Handbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4From EverandHandbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4Rating: 5 out of 5 stars5/5 (1)
- Mass LabDocument13 pagesMass Labhagt813No ratings yet
- Birla Copper WPS For Tube To Tubesheet Welding MOC - CSDocument2 pagesBirla Copper WPS For Tube To Tubesheet Welding MOC - CSJitu Padhiyar PadhiyarNo ratings yet
- Belzona 4311: Instructions For UseDocument2 pagesBelzona 4311: Instructions For UseAnsar AliNo ratings yet
- Acacia ReactorDocument11 pagesAcacia ReactorPedrin Cha OlivosNo ratings yet
- Bacteria Count LabsheetDocument7 pagesBacteria Count LabsheetAbby Faiz100% (1)
- Presentation of Damei KingmechDocument86 pagesPresentation of Damei Kingmechbambang sutrimoNo ratings yet
- Ignition Probability PDFDocument12 pagesIgnition Probability PDFmaha_specialsNo ratings yet
- Bead and MeshDocument7 pagesBead and Meshmuradali01No ratings yet
- Datasheet IBC FlexiSunDocument2 pagesDatasheet IBC FlexiSunfarani87No ratings yet
- ORD 5712 Parker O-Ring Material Offering GuideDocument77 pagesORD 5712 Parker O-Ring Material Offering GuideCaio ZeredoNo ratings yet
- Exp 1 Vinegar Sample ReportDocument4 pagesExp 1 Vinegar Sample Reportmuhammad aliNo ratings yet
- Experiment: Factors Affecting Rate of ReactionsDocument10 pagesExperiment: Factors Affecting Rate of ReactionsAnonymous BPFqriLDNo ratings yet
- 577 Iso Fdis 17225-2Document15 pages577 Iso Fdis 17225-2order.memorywaterNo ratings yet
- Chapter 4Document35 pagesChapter 4Leon TanNo ratings yet
- 17-03-14 Controlling Fire and Explosion Risk StandardDocument8 pages17-03-14 Controlling Fire and Explosion Risk StandardvengielNo ratings yet
- Chilled BeamDocument16 pagesChilled BeamDEVIKA ANILNo ratings yet
- Chemistry Book ProjectDocument80 pagesChemistry Book ProjectbaymaxNo ratings yet
- Perfume Industry AssignmentDocument20 pagesPerfume Industry AssignmentHajar Hadis50% (2)
- Drug Receptor InteractionsDocument29 pagesDrug Receptor InteractionscsujithaNo ratings yet
- Compact Ring-Torsion Load Cells RTNDocument4 pagesCompact Ring-Torsion Load Cells RTNAbdul Moeez AliNo ratings yet
- Geography ProjectDocument7 pagesGeography Projectsachin DesardaNo ratings yet
- Freitag Method To Determine UV StabilizerDocument2 pagesFreitag Method To Determine UV Stabilizernazrul islamNo ratings yet
- SOP For Automatic Batch Printing MachineDocument5 pagesSOP For Automatic Batch Printing MachineAshok KumarNo ratings yet
- Language Worksheet 1 Vocabulary BuildingDocument2 pagesLanguage Worksheet 1 Vocabulary BuildingGenna EvelloNo ratings yet
- HEI-IMS-CHEM-SOP06 Chemicals Handling ProcedureDocument22 pagesHEI-IMS-CHEM-SOP06 Chemicals Handling Procedurerizwan chNo ratings yet
- Check List Regarding Documents Submitted For Drug Registration PurposesDocument8 pagesCheck List Regarding Documents Submitted For Drug Registration PurposesMayson Bali100% (1)
- Bio NoteDocument5 pagesBio NoteErikNo ratings yet
- Repair of CO Stripper Column Top Head: D. G. Damin and L. W. Ridenhour, JRDocument7 pagesRepair of CO Stripper Column Top Head: D. G. Damin and L. W. Ridenhour, JRvaratharajan g rNo ratings yet
- Review of Related Literature and StudiesDocument4 pagesReview of Related Literature and StudiesArjay Verdejo50% (2)
- Majorsol CottonDocument5 pagesMajorsol CottonAries Agro LimitedNo ratings yet