Professional Documents
Culture Documents
Test PDF
Uploaded by
Cory Wood0 ratings0% found this document useful (0 votes)
5 views1 pageThis document discusses an experiment to verify the relationship between light frequency, electron stopping potential, and metal work function using the photoelectric effect. The energy of photons is equal to Planck's constant times the frequency. Electrons in metals are bound by a work function energy. The kinetic energy of emitted electrons equals the photon energy minus the work function. The experiment uses a photocell, ammeter, and voltage source to measure current from emitted electrons when illuminated with light of varying frequencies.
Original Description:
Original Title
Test.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document discusses an experiment to verify the relationship between light frequency, electron stopping potential, and metal work function using the photoelectric effect. The energy of photons is equal to Planck's constant times the frequency. Electrons in metals are bound by a work function energy. The kinetic energy of emitted electrons equals the photon energy minus the work function. The experiment uses a photocell, ammeter, and voltage source to measure current from emitted electrons when illuminated with light of varying frequencies.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views1 pageTest PDF
Uploaded by
Cory WoodThis document discusses an experiment to verify the relationship between light frequency, electron stopping potential, and metal work function using the photoelectric effect. The energy of photons is equal to Planck's constant times the frequency. Electrons in metals are bound by a work function energy. The kinetic energy of emitted electrons equals the photon energy minus the work function. The experiment uses a photocell, ammeter, and voltage source to measure current from emitted electrons when illuminated with light of varying frequencies.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
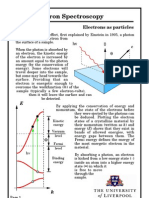
1 Introduction and Theory
When light shines on a metal surface, electrons are found to be emitted from the
surface. This phenomenon is called the photoelectric effect. In this experiment
we want to verify the relationship between the frequency of the incident light,
stopping potential for the liberated electrons, and the work function of the
metal. To accomplish this we will utilize the photoelectric effect to measure the
ratio of Planck’s constant and the electron charge.
The energy of a photon is given by the famous equation
E = hf,
where E is the energy, h is Planck’s constant, f is the frequency of the photon.
Consider the electrons in the metal as being bound in a potential. The binding
energy is called the work function, W0 . Using conservation of energy, The
kinetic energy of the liberated electrons is the difference between the energy of
the incident photon and the work function of the metal:
1
me v 2 = hf − W0 .
2
A common experimental setup for observing the photoelectric effect is to
have a photocell connected to a ammeter and a source of emf (as described more
so in the next section). when the photocell is in the dark (no light shinning on
it), the ammeter reads zero. But when light of a sufficiently high frequency
illuminates the photocell the ammeter indicates a current flowing within the
circuit.
You might also like
- Cape Physics Module 3Document30 pagesCape Physics Module 3Brandon Gurrell100% (1)
- Advanced Physics Laboratory XRF X-Ray Fluorescence: Energy-Dispersive Analysis (EDXRF)Document14 pagesAdvanced Physics Laboratory XRF X-Ray Fluorescence: Energy-Dispersive Analysis (EDXRF)Gandis YulianaNo ratings yet
- Photo-Electric Effect and ComptonDocument7 pagesPhoto-Electric Effect and ComptonMohammad Irfan YousufNo ratings yet
- Photoelectric EffectDocument13 pagesPhotoelectric EffectEr Purushottam PalNo ratings yet
- PHYSICS 286: Modern Physics Laboratory Experiment 6: The Photoelectric EffectDocument6 pagesPHYSICS 286: Modern Physics Laboratory Experiment 6: The Photoelectric EffectAashirwadNo ratings yet
- Factsheet Physics: The Quantum Nature of LightDocument6 pagesFactsheet Physics: The Quantum Nature of LightddsddsddsNo ratings yet
- Quantization of LightDocument12 pagesQuantization of LightPRIYAA A/P JAYASANKAR / UPMNo ratings yet
- Factsheet Physics: The Quantum Nature of LightDocument6 pagesFactsheet Physics: The Quantum Nature of Lightpwebb661919No ratings yet
- Photoelectric EffectDocument5 pagesPhotoelectric EffectUsman GhaniNo ratings yet
- Photoelectric EffectDocument1 pagePhotoelectric EffectA.SheikhNo ratings yet
- Photoelectric Effect ProblemsDocument1 pagePhotoelectric Effect ProblemsaminulahsanNo ratings yet
- Manan Vyas - XII A2Document14 pagesManan Vyas - XII A2samplghNo ratings yet
- Photoelectric EffectDocument6 pagesPhotoelectric EffectGlay Cabañero TacanayNo ratings yet
- PhotoelectricDocument6 pagesPhotoelectricNgọc DươngNo ratings yet
- CH 16 - Quantam PhysicsDocument45 pagesCH 16 - Quantam PhysicsOKAY JAROD PUGNo ratings yet
- Photoelectron Spectroscopy: Electrons As ParticlesDocument2 pagesPhotoelectron Spectroscopy: Electrons As ParticlesRaees Ahmad100% (1)
- Pedrotti - Bab 6 - LASERDocument32 pagesPedrotti - Bab 6 - LASERTheresia AnggitaNo ratings yet
- 29 Particles and WavesDocument14 pages29 Particles and WavesMikaela Rome BigayNo ratings yet
- 12.1 Lab Photoelectric Effect-1-3Document3 pages12.1 Lab Photoelectric Effect-1-3Bhavya BhattNo ratings yet
- 11-Dual NatureDocument5 pages11-Dual NatureAyushi KapoorNo ratings yet
- Photo Electric EffectDocument19 pagesPhoto Electric EffectAbdullah ZafarNo ratings yet
- QuantumDocument15 pagesQuantumBoedisantosoNo ratings yet
- Photoelectric EffectDocument5 pagesPhotoelectric EffectRoxy TudorNo ratings yet
- Quantum Physics (1) A LEVELDocument21 pagesQuantum Physics (1) A LEVELkhodabuccuswal7No ratings yet
- 11 - Efecto Fotoeléctrico 2023Document19 pages11 - Efecto Fotoeléctrico 2023leiderNo ratings yet
- Chapter 3 Photoelectric Effect2Document40 pagesChapter 3 Photoelectric Effect2moxsueNo ratings yet
- ComptonDocument10 pagesComptonnvknsharmaNo ratings yet
- FORM 5 Chapter 7 Quantum PhysicsDocument4 pagesFORM 5 Chapter 7 Quantum PhysicsKishenraj RajendranNo ratings yet
- Photo Electric Effect NotesDocument11 pagesPhoto Electric Effect NotesChitransh AwasthiNo ratings yet
- 19 Quantum Physics Summary PDFDocument2 pages19 Quantum Physics Summary PDFabdul halimNo ratings yet
- Photoelectric EffectDocument14 pagesPhotoelectric EffectTamilselvan BaskaranNo ratings yet
- Dual Nature of Light Cbse NotesDocument11 pagesDual Nature of Light Cbse Notesrchandra2473No ratings yet
- 1 Basics of Optical Emission and AbsorptionDocument10 pages1 Basics of Optical Emission and AbsorptionChuxuan SunNo ratings yet
- Photoelectric Effect:-: Electrons. This Phenomenon Is Commonly Studied in Electronic Physics, As Well AsDocument16 pagesPhotoelectric Effect:-: Electrons. This Phenomenon Is Commonly Studied in Electronic Physics, As Well AsSourav Paul100% (1)
- Photoelectric Effect PDFDocument26 pagesPhotoelectric Effect PDFSabbirNo ratings yet
- 4.5. Quantum PhysicsDocument4 pages4.5. Quantum PhysicsjmsonlNo ratings yet
- Electromagnetic Radiation PDFDocument2 pagesElectromagnetic Radiation PDFMuhammadBilalNo ratings yet
- CONTENTDocument9 pagesCONTENTAshutosh KumarNo ratings yet
- Quantum Physics Notes CWDocument14 pagesQuantum Physics Notes CWFatimah AfzalNo ratings yet
- Phy Jan's ProjectDocument34 pagesPhy Jan's ProjectSakthiiNo ratings yet
- 2013 H2 Physics Tut18 Quantum Physics Part1 (Solutions)Document14 pages2013 H2 Physics Tut18 Quantum Physics Part1 (Solutions)Wee Chee LimNo ratings yet
- 02 IntroQuantumPhysicsDocument8 pages02 IntroQuantumPhysicscychan410No ratings yet
- Where As Strength of Conventional Light Would Not Exceed 10: Nonlinear OpticsDocument4 pagesWhere As Strength of Conventional Light Would Not Exceed 10: Nonlinear OpticsSatish GhelaniNo ratings yet
- Photoelectric EffectDocument3 pagesPhotoelectric EffectFilip Leonard100% (1)
- Expt. 3 Planck's ConstantDocument10 pagesExpt. 3 Planck's ConstantSandeepNo ratings yet
- Dual Nature of Matter and RadiationDocument6 pagesDual Nature of Matter and RadiationGayatriNo ratings yet
- Photo Electric Effect Ho 09 Jan EngDocument15 pagesPhoto Electric Effect Ho 09 Jan EngPsatis PatelNo ratings yet
- Physics Presentation On Photon.Document18 pagesPhysics Presentation On Photon.anjaltiwari90No ratings yet
- Atomic and Nuclear Physics: Determining Planck's ConstantDocument4 pagesAtomic and Nuclear Physics: Determining Planck's ConstantAlejandra AwimbaweNo ratings yet
- Photo-Electric Effect and ComptonDocument7 pagesPhoto-Electric Effect and ComptonsumiNo ratings yet
- All India Senior Secondary School Certificate Examination (AISSCE-2013-14)Document10 pagesAll India Senior Secondary School Certificate Examination (AISSCE-2013-14)ScientistNo ratings yet
- Photoelectric EffectDocument10 pagesPhotoelectric EffectScientistNo ratings yet
- Maximum: LightingDocument1 pageMaximum: Lightingreacharunk100% (1)
- Atomic Physics 2.photoelectric Effect Points To RememberDocument10 pagesAtomic Physics 2.photoelectric Effect Points To RememberMAHESH DNo ratings yet
- Atomic Physics 2.photoelectric Effect Points To RememberDocument10 pagesAtomic Physics 2.photoelectric Effect Points To RememberMAHESH DNo ratings yet
- QPhysics-Lab 1 - Photoelectric EffectDocument2 pagesQPhysics-Lab 1 - Photoelectric EffectEvariste MigaboNo ratings yet
- Simplest Working Example LaTeX Document 1Document3 pagesSimplest Working Example LaTeX Document 1rishikaNo ratings yet
- Photoelectric EffectDocument11 pagesPhotoelectric EffectRivu BasuNo ratings yet
- Dual Nature of Radiation and Matter: Can You Recall?Document35 pagesDual Nature of Radiation and Matter: Can You Recall?RAVINDRA WAYKOLENo ratings yet
- Index NotationDocument10 pagesIndex Notationnavy12347777No ratings yet
- DevPo StoryDocument6 pagesDevPo StoryCory WoodNo ratings yet
- Test PDFDocument1 pageTest PDFCory WoodNo ratings yet
- Test PDFDocument2 pagesTest PDFCory WoodNo ratings yet
- Final Exam: Professor Uehara April 2019Document1 pageFinal Exam: Professor Uehara April 2019Cory WoodNo ratings yet
- Final Exam: Professor Uehara April 2019Document1 pageFinal Exam: Professor Uehara April 2019Cory WoodNo ratings yet