0% found this document useful (0 votes)
304 views14 pagesBasic Thermal Engineering (ME3100) : Pallab Sinha Mahapatra
This document provides an overview of basic thermal engineering concepts including:
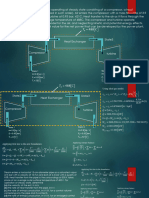
- The first law of thermodynamics for control volumes and work transfer equations for turbines and compressors.
- Equations for calculating isentropic work in compression and expansion processes.
- Properties of steam and examples of calculating steam quality and velocity after expansion through turbines.
- Equations for calculating enthalpy of compressed liquid and obtaining properties at saturation temperature.
Uploaded by
AMAN GAUTAMCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
304 views14 pagesBasic Thermal Engineering (ME3100) : Pallab Sinha Mahapatra
This document provides an overview of basic thermal engineering concepts including:
- The first law of thermodynamics for control volumes and work transfer equations for turbines and compressors.
- Equations for calculating isentropic work in compression and expansion processes.
- Properties of steam and examples of calculating steam quality and velocity after expansion through turbines.
- Equations for calculating enthalpy of compressed liquid and obtaining properties at saturation temperature.
Uploaded by
AMAN GAUTAMCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd