Professional Documents
Culture Documents
Tugas Science and Enginerring1
Tugas Science and Enginerring1
Uploaded by
Afif Fauzan MOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tugas Science and Enginerring1
Tugas Science and Enginerring1
Uploaded by
Afif Fauzan MCopyright:
Available Formats
Name: Afif Fauzan Muslim
Student ID: 17951026
Topic: Rare Earth Element (REE)
The rare earth elements have long been recognised as useful because of their unusual chemical and
physical properties. Their natural occurrence is strongly dependent on geological circumstances, and
only in a few locations are they found in sufficient quantity and concentration, and in a suitable form
and setting, to make their extraction and exploitation economically viable. The rare earth elements
have also been of longstanding interest to geologists as tools for furthering scientific research into
the origins of rocks and ores and into the chemical behaviour of ocean waters.
What are the rare earth elements?
The rare earth elements (REE) are a group of seventeen metallic elements - the fifteen lanthanides,
with atomic numbers 57 (lanthanum, La) to 71 (lutetium, Lu), together with yttrium (Y, atomic
number 39) and scandium (Sc, atomic number 21). All have similar chemical properties. The lower
atomic weight elements lanthanum to samarium (Sm), with atomic numbers 57 to 62, are referred to
as the light rare earth elements (LREE); while europium (Eu) to lutetium, with atomic numbers 63 to
71, are the heavy rare earth elements (HREE). (The dividing line drawn between LREE and HREE can
vary somewhat, and the term ‘mid REE’ is also now sometimes used.) Yttrium, although it has a
lower atomic weight, is grouped with the HREE because of its chemical similarity. Scandium’s
properties are different enough from those of the other REE that most of the scientific and general
literature excludes it and focuses on the lanthanides and yttrium
How rare are they?
The term ‘rare earths’ was first used in the late eighteenth and early nineteenth centuries, to refer to
minerals containing REE and some other metals, known deposits of which were rare – only
subsequently did the name ‘rare earth elements’ come to be associated solely with the specific set of
elements it denotes today. In fact, in terms of their overall abundance in the Earth’s crust, the REE
are not particularly rare. On average, as a proportion of the Earth’s continental crust, Cerium (Ce) is
the most abundant, at 43 parts per million (ppm), followed by lanthanum (20 ppm) and neodymium
(Nd, 20 ppm). The rarest REE is thulium (Tm, 0.28 ppm), except for promethium (Pm) which is
virtually absent, since it is radioactive with a short half-life. Yttrium occurs at 19 ppm. So their overall
abundances are not dissimilar from many other important elements such as lithium (17 ppm),
germanium (1.3 ppm), copper (27 ppm), tin (1.7 ppm) and uranium (1.3 ppm).
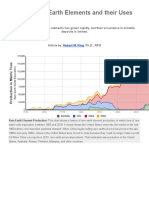
What are the uses of rare earth elements?
With new and developing technologies, the uses of REE have extended from well-established
applications such as glass polishing, to include high-performance magnets, high-tech catalysts,
electronics, glass, ceramics, and alloys. An increasingly important area of REE use is in low-carbon
technologies. The main applications of REE are shown in the table
Magnets Catalysts Alloys Glass and Miscellaneous
Electronics
Examples Electric motors in Petroleum NiMH Display Ceramics;
of use hybrid vehicles; refining; batteries; Fuel phosphors Water
Wind power Catalytic cells; Other (compact treatment;
generators; Hard converters; alloys (with fluorescent Nuclear fuel
disc drives; CD and Diesel iron, lamps, LCD rods;
DVD players; additives. magnesium, and plasma Pigments;
Imaging; Portable aluminium screens, Fertiliser;
electronics; and in special cathode ray Medical
Microphones and steels). tubes. tracers.
You might also like
- FAQ On Rare Earths ElementsDocument4 pagesFAQ On Rare Earths ElementsVeeramani ArumugamNo ratings yet
- Rare Eaths DescripcionDocument10 pagesRare Eaths DescripcionRicardo JesusNo ratings yet
- Mineralogi Lateritic NickelDocument56 pagesMineralogi Lateritic NickelMilky LalisaNo ratings yet
- Business of Rare Earth ElementsDocument6 pagesBusiness of Rare Earth ElementsAnanthNo ratings yet
- Rare Earth ElementsDocument41 pagesRare Earth ElementsBobNo ratings yet
- Rare-Earth ElementDocument31 pagesRare-Earth Elementtrojan_horse13No ratings yet
- Rare Earth Elements: Procurement, Application, and ReclamationDocument32 pagesRare Earth Elements: Procurement, Application, and ReclamationDouglas YusufNo ratings yet
- REE - A Brief OverviewDocument15 pagesREE - A Brief OverviewArfinsa AinurzanaNo ratings yet
- IJCCE - Volume 26 - Issue 4 - Pages 11-18Document8 pagesIJCCE - Volume 26 - Issue 4 - Pages 11-18nazanin timasiNo ratings yet
- Rare Earth Minerals and ClassificationDocument10 pagesRare Earth Minerals and Classificationnasir.hdip8468No ratings yet
- Rare-Earth ElementDocument12 pagesRare-Earth ElementchristopheNo ratings yet
- REE - Rare Earth Elements: Home MetalsDocument10 pagesREE - Rare Earth Elements: Home MetalsMorkizgaNo ratings yet
- Dushyantha Et Al., 2020Document17 pagesDushyantha Et Al., 2020gigio marino100% (1)
- Extraction of Metals From E-Waste Seminar Report, 2016Document27 pagesExtraction of Metals From E-Waste Seminar Report, 2016aromal josephNo ratings yet
- Environmental and Social Challenges of The: Mining of Critical MetalsDocument19 pagesEnvironmental and Social Challenges of The: Mining of Critical MetalsAnn MariyaNo ratings yet
- Name: Sunena R.N College: S.N.G.K B. Ed College Subject: Physical ScienceDocument10 pagesName: Sunena R.N College: S.N.G.K B. Ed College Subject: Physical ScienceRaghul CRNo ratings yet
- Gospo 2016 0039Document16 pagesGospo 2016 0039Saman FatimaNo ratings yet
- Research Article: Life Cycle Impact of Rare Earth ElementsDocument11 pagesResearch Article: Life Cycle Impact of Rare Earth ElementsAleenaNo ratings yet
- Rare EarthDocument10 pagesRare Earthjagadish.kvNo ratings yet
- Rare Earth Element: IntroductionDocument15 pagesRare Earth Element: IntroductionNibin TomNo ratings yet
- Byron Capital Markets Rare Earths Industry ReportDocument20 pagesByron Capital Markets Rare Earths Industry Reportgeorg1967No ratings yet
- Chapter 2: Literature ReviewDocument67 pagesChapter 2: Literature Reviewpravin kondeNo ratings yet
- REE Rare EarthsDocument11 pagesREE Rare EarthsRicardo JesusNo ratings yet
- Role of Critical Metals in The Future Markets of Clean Energy Technologiesrenewable EnergyDocument10 pagesRole of Critical Metals in The Future Markets of Clean Energy Technologiesrenewable EnergyDouglas SantosNo ratings yet
- What Happens After The Rare Earth Crisis: A Systematic Literature ReviewDocument26 pagesWhat Happens After The Rare Earth Crisis: A Systematic Literature ReviewAdinIhtisyamuddinNo ratings yet
- Characterizing and Recovering The Platinum Group Minerals - A ReviewDocument19 pagesCharacterizing and Recovering The Platinum Group Minerals - A ReviewSlim Kat NkosiNo ratings yet
- Applications of LN & Ac.Document6 pagesApplications of LN & Ac.Amna NasirNo ratings yet
- Thorium Resources in Rare Earth Elements (Ragheb, M., Aug. 2011Document42 pagesThorium Resources in Rare Earth Elements (Ragheb, M., Aug. 2011kalyan974696No ratings yet
- Rare Earth ElementsDocument8 pagesRare Earth ElementsAnshuman TagoreNo ratings yet
- Binnemans2013 PDFDocument22 pagesBinnemans2013 PDFJenniGaticaNo ratings yet
- Handbook of RE and Alloys (P, E, Pre, App)Document13 pagesHandbook of RE and Alloys (P, E, Pre, App)yeyintlayNo ratings yet
- Geothermics: SciencedirectDocument9 pagesGeothermics: Sciencedirectsasha1520No ratings yet
- Based Noble: Electrodes MetalsDocument10 pagesBased Noble: Electrodes MetalsSumedh WaradeNo ratings yet
- 1 s2.0 S0038092X17310162 MainDocument12 pages1 s2.0 S0038092X17310162 Mainlucas italoNo ratings yet
- Nano CuODocument9 pagesNano CuOcanilkumarrichithaNo ratings yet
- (2021) Ultrasonic Coal Fly AshDocument29 pages(2021) Ultrasonic Coal Fly AshMincen RevaNo ratings yet
- REE in The DRCDocument19 pagesREE in The DRCJames MunagaNo ratings yet
- Metals: Research Progress in Preparation and Purification of Rare Earth MetalsDocument13 pagesMetals: Research Progress in Preparation and Purification of Rare Earth MetalsmtanaydinNo ratings yet
- Earth Science OutputsDocument7 pagesEarth Science OutputsRangerbackNo ratings yet
- Afghanistan MineralsDocument101 pagesAfghanistan MineralsKhan MohammadNo ratings yet
- NanoparticlesDocument13 pagesNanoparticlesArslan JigarNo ratings yet
- Universiti Tenaga Nasional: Progress Report 1Document18 pagesUniversiti Tenaga Nasional: Progress Report 1Tengku AsyrafNo ratings yet
- Heavy Metal Water Pollution-A Case Study: Recent Research in Science and Technology 2013, 5 (5) : 98-99 ISSN: 2076-5061Document2 pagesHeavy Metal Water Pollution-A Case Study: Recent Research in Science and Technology 2013, 5 (5) : 98-99 ISSN: 2076-5061Zari Sofia LevisteNo ratings yet
- REE Market PaperDocument21 pagesREE Market PaperAbie BadhurahmanNo ratings yet
- MoSe2 PEMelectrolysis CorralesDocument14 pagesMoSe2 PEMelectrolysis CorralesleonardoNo ratings yet
- 1 s2.0 S1002072120304762 MainDocument8 pages1 s2.0 S1002072120304762 MainIndriani NoviagelNo ratings yet
- Aachen Presentation On EudialyteDocument20 pagesAachen Presentation On Eudialytejiawen dingNo ratings yet
- Gold Heavy Metal SensorDocument8 pagesGold Heavy Metal SensorSurinder SinghNo ratings yet
- University of Northeastern Philippines: Iriga City, Camarines SurDocument31 pagesUniversity of Northeastern Philippines: Iriga City, Camarines SurZudotaNo ratings yet
- Examples of Nano Materials (1-3)Document9 pagesExamples of Nano Materials (1-3)Joshua Iver OrolazaNo ratings yet
- General Chemistry Script and FlowDocument7 pagesGeneral Chemistry Script and FlowdecastroghislaneNo ratings yet
- CRT Pyrometallurgical Hydrometallurgical e WasteDocument33 pagesCRT Pyrometallurgical Hydrometallurgical e WastesonficyusNo ratings yet
- The Role and Challenges of Rare Earths in The Energy TransitionDocument41 pagesThe Role and Challenges of Rare Earths in The Energy TransitionYASSINE KIRATNo ratings yet
- Electrochimica Acta: Karthikeyan Krishnamoorthy, Parthiban Pazhamalai, Sang Jae KimDocument10 pagesElectrochimica Acta: Karthikeyan Krishnamoorthy, Parthiban Pazhamalai, Sang Jae KimkarthikNo ratings yet
- Rare Earths 101: Rare Earth Elements and The Green Energy EconomyDocument6 pagesRare Earths 101: Rare Earth Elements and The Green Energy Economyjj831983No ratings yet
- Rare Earth ElementsDocument34 pagesRare Earth ElementsAgnidipto BasuNo ratings yet
- ReviewArticle PDFDocument7 pagesReviewArticle PDFwaleligneNo ratings yet
- Unit 1-Chapter 1. Introduction To Engg. MaterialsDocument76 pagesUnit 1-Chapter 1. Introduction To Engg. Materialssainath reddy kesam reddyNo ratings yet
- WP XRF Analyzing Precious MetalsDocument15 pagesWP XRF Analyzing Precious MetalsVikin JainNo ratings yet
- PD Medical Certification PD 615ADocument3 pagesPD Medical Certification PD 615AAfif Fauzan MNo ratings yet
- Ohayou GozaimasuDocument1 pageOhayou GozaimasuAfif Fauzan MNo ratings yet
- Why Need To Count Soil SuctionDocument1 pageWhy Need To Count Soil SuctionAfif Fauzan MNo ratings yet
- Challenges: - Inadequate Resources, Difficult To Get Vegetables, LackDocument9 pagesChallenges: - Inadequate Resources, Difficult To Get Vegetables, LackAfif Fauzan MNo ratings yet
- Koreksi Sudut (N - 2) - 180Document6 pagesKoreksi Sudut (N - 2) - 180Afif Fauzan MNo ratings yet
- Comment and Essay To The Presentation From Noor Octavian Anwar and Jeon Soo Young About Do You Enjoy Your Major?Document1 pageComment and Essay To The Presentation From Noor Octavian Anwar and Jeon Soo Young About Do You Enjoy Your Major?Afif Fauzan MNo ratings yet
- 1 Eksplorasi Geoffrey de Jong PTFI Prosiding FixDocument7 pages1 Eksplorasi Geoffrey de Jong PTFI Prosiding FixRidwan KotoNo ratings yet
- 5070 s09 QP 1 PDFDocument16 pages5070 s09 QP 1 PDFNeural Spark Physics CieNo ratings yet
- Ds Project ReportDocument22 pagesDs Project ReportSamyuktha PatlollaNo ratings yet
- Atomic Number Element SymbolDocument4 pagesAtomic Number Element SymbolPaneyNo ratings yet
- Chemsitry Past Papers June 2003 - Paper 1Document16 pagesChemsitry Past Papers June 2003 - Paper 1theyaasir100% (1)
- List of Inorganic Compounds - WikipediaDocument93 pagesList of Inorganic Compounds - WikipediaSushil kumar NagNo ratings yet
- List of Oxidation States of The Elements PDFDocument5 pagesList of Oxidation States of The Elements PDFThe ChampionNo ratings yet
- Star Control: Origins Alien Locations & MoreDocument13 pagesStar Control: Origins Alien Locations & MoreTommy CashmanNo ratings yet
- 0620 s15 QP 13 PDFDocument16 pages0620 s15 QP 13 PDFNgoc Quang NguyenNo ratings yet
- Typical Gain or EHT Values For AA Hollow Cathode Lamps PDFDocument3 pagesTypical Gain or EHT Values For AA Hollow Cathode Lamps PDFZaenudin 28No ratings yet
- Tensile Bond TestDocument14 pagesTensile Bond TestPanneer SelvamNo ratings yet
- Elements in Our Body and in TechnologyDocument46 pagesElements in Our Body and in TechnologySherilyn Closa BunagNo ratings yet
- LanthanidesDocument24 pagesLanthanidesJCkarlos1No ratings yet
- OREAS 620: Volcanic Hosted Massive Sulphide Zn-Pb-Cu-Ag-Au Ore Certified Reference MaterialDocument17 pagesOREAS 620: Volcanic Hosted Massive Sulphide Zn-Pb-Cu-Ag-Au Ore Certified Reference MaterialKEVINNo ratings yet
- Towards Cleaner Production of Rare Earth Elements From Bastnaesite in ChinaDocument12 pagesTowards Cleaner Production of Rare Earth Elements From Bastnaesite in ChinaHoracio Piña SpeziaNo ratings yet
- 0620 w17 QP 12 PDFDocument16 pages0620 w17 QP 12 PDFyuke kristinaNo ratings yet
- Tableofelements PDFDocument1 pageTableofelements PDFmallika29No ratings yet
- AA-7000 Series Consumables: Atomic Absorption SpectrophotometersDocument17 pagesAA-7000 Series Consumables: Atomic Absorption SpectrophotometersCarlos Eduardo FreuNo ratings yet
- Keterangan Unsur Kimia Di Tabel PeriodikDocument5 pagesKeterangan Unsur Kimia Di Tabel PeriodikAndira SalsabilaNo ratings yet
- The Separation of Rare Earths by Ion Exchange. 1. Cerium and YttriumDocument5 pagesThe Separation of Rare Earths by Ion Exchange. 1. Cerium and Yttriumyomister1No ratings yet
- Homogeneous Catalysts. Types, Reactions and ApplicationsDocument528 pagesHomogeneous Catalysts. Types, Reactions and ApplicationsLuis Mauricio Contreras100% (2)
- CRM List 2020 - Final ReportDocument158 pagesCRM List 2020 - Final ReportsseemaNo ratings yet
- Bolt MaterialDocument817 pagesBolt MaterialTombongNo ratings yet
- Magnesium Alloys Containing Rare Earth MetalsDocument27 pagesMagnesium Alloys Containing Rare Earth Metalsoğuz kağanNo ratings yet
- IJCCE - Volume 26 - Issue 4 - Pages 11-18Document8 pagesIJCCE - Volume 26 - Issue 4 - Pages 11-18nazanin timasiNo ratings yet
- Msds PorcelainDocument19 pagesMsds PorcelainIPKL RSBHAYANGKARA KEDIRINo ratings yet
- Non-Ferrous Metals..Presentation of Emat...Document26 pagesNon-Ferrous Metals..Presentation of Emat...Naveed AhmedNo ratings yet
- History of The Periodic Table QuestionsDocument4 pagesHistory of The Periodic Table QuestionsFlorinda GagasaNo ratings yet