Professional Documents
Culture Documents
US6476128
Uploaded by
desipoetOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
US6476128
Uploaded by
desipoetCopyright:
Available Formats
USOO6476128B1
(12) United States Patent (10) Patent No.: US 6,476,128 B1
Berzinis (45) Date of Patent: Nov. 5, 2002
(54) LOW-GLOSS BLENDS CONTAINING POLY (56) References Cited
(METH)ACRYLATE RUBBER-BASED GRAFT
COPOLYMER AND PROCESS FOR MAKING U.S. PATENT DOCUMENTS
THEREOF
5,441,816 A 8/1995 Grohman .................... 428/520
(75) Inventor: Albin P. Berzinis, Marietta, OH (US) * cited by examiner
(73) Assignee: General Electric Company, Pittsfield,
MA (US) Primary Examiner James J. Seidleck
Assistant Examiner-Olga Asinovsky
Notice: Subject to any disclaimer, the term of this (57) ABSTRACT
patent is extended or adjusted under 35
U.S.C. 154(b) by 0 days. A proceSS for controlling the Surface gloSS of a molded
composition of: (a) a polyvinyl chloride resin and (b) a graft
(21) Appl. No.: 09/822,667 copolymer comprising a discontinuous poly(alkyl(meth)
(22) Filed: Mar. 30, 2001 acrylate) rubber phase and a rigid thermoplastic phase,
wherein at least a portion of rigid thermoplastic phase is
(51) Int. Cl." ......................... C08L 51/04; CO8L 33/06; chemically grafted to the poly(alkyl(meth)acrylate) rubber
CO8F 259/04 phase, by regulating the croSS-link density and thus the Swell
(52) U.S. Cl. ............................. 525/70; 525/76; 525/80; index of the rubber phase in the graft copolymer to at least
525/222; 525/317 about 8 to vary the Surface gloSS after extrusion.
(58) Field of Search .............................. 525/70, 80, 76,
525/222,317 11 Claims, 2 Drawing Sheets
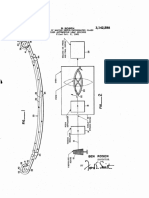
U.S. Patent Nov. 5, 2002 Sheet 1 of 2 US 6,476,128 B1
X
O)
O
Q)
O)
C
>
O
CD
b)
o
"O
p
(f)
seesep GA, ess of 3upS
U.S. Patent Nov. 5, 2002 Sheet 2 of 2 US 6,476,128 B1
>
O)
A
M
O
O
A
>
Z
t
>
O
CD
b)
e
r
U?) C O C O O O
SC O r cr) CN w
seesep GA. Jessor upS
US 6,476,128 B1
1 2
LOW-GLOSS BLENDS CONTAINING POLY in the emulsion polymerization reaction to prepare the
(METH)ACRYLATE RUBBER-BASED GRAFT poly(alkyl(meth)acrylate) rubber phase.
COPOLYMER AND PROCESS FOR MAKING In a Second embodiment of the present invention, the
THEREOF polyethyleneically unsaturated monomer which functions as
the cross-linking and graft-linking monomer in the prepa
FIELD OF THE INVENTION
ration of the poly(alkyl(meth)acrylate) rubber latex com
prises triallyl cyanurate.
The present invention relates to a proceSS for controlling BRIEF DESCRIPTION OF THE DRAWINGS
the Siding gloSS in thermoplastic compositions comprising a 1O
blend of PVC resin and ASA graft copolymers. FIG. 1 shows a plot of siding gloss versus Swell index of
BACKGROUND
the graft copolymer in the process of the present invention.
FIG. 2 shows a plot of siding gloss versus the NMR
Graft copolymers comprising a discountinous poly(alkyl croSS-link density of the graft copolymer in the process of
(meth)acrylate rubber phase and a rigid thermoplastic phase, 15 the present invention.
e.g., ASA graft copolymers, may be used in extrusion
compositions for their desirable properties as impact modi DETAILED DESCRIPTION OF THE
fiers. U.S. Pat. No. 6,054,531 discloses a method for con INVENTION
trolling the Swell indeX and gel content of an emulsion
polymerized croSS-linked polyacrylate rubber to improve its The present invention involves, in one of its aspects, a
performance as an impact modifier in a thermoplastic mate thermoplastic composition comprising a polyvinyl chloride
rial. EP Publication No. 913408 discloses a process for (PVC) and a graft copolymer.
making a polyacrylate rubber in the presence of a Surfactant A. PVC for the Composition
having the formula R-SOM wherein R is an alkyl, and M The first component of the low-gloSS extrusion composi
is a hydrogen radical or a cation. Such a rubber is found to 25 tion of the present invention is polyvinyl chloride or PVC.
provide improved weatherability when used as an impact PVC polymer as used in this invention means polyvinyl
modifier in thermoplastic resin extrusion compositions. chloride homopolymers, or vinyl chloride polymerized with
Thermoplastic compositions comprising graft copolymers up to 50% by weight of one or more other monomer(s), or
are often used in weatherable applications Such as outdoor a croSS-linked polyvinyl chloride polymer, or mixtures
furniture or housing Sidings. It is often desirable to control thereof. The PVC polymer of this invention may be pro
the gloSS of the Surface of Such articles to improve duced by any of the known polymerization processes Such as
aesthetics, e.g., for the Sidings to have more of a wood-like mass, Suspension, Solution or emulsion.
appearance. One practice is to add abrasive fillers such as In one embodiment of the present invention, the polyvinyl
aluminum Silicate or calcium carbonate to the thermoplastic chloride polymer is a polyvinyl chloride homopolymer.
compositions to give a matte surface. U.S. Pat. No. 4,945, 35 In another embodiment of the invention, PVC copolymers
131 teaches the regulation of the Swell index of high impact are used. Comonomers that may be used to give a PVC
polystyrene rubber phase to control the Surface gloSS of a copolymer includes esters of acrylic acid, for example,
blend of polyphenylene ether resin after molding. Going the methyl acrylate, ethyl acrylate, butyl acrylate, octyl acrylate,
reverse direction with respect to the effect of croSS-linking cyanoethyl acrylate, and the like; Vinyl acetate, esters of
on surface gloss, U.S. Pat. No. 5,130,374 discloses thermo 40 methacrylic acid, Such as methyl methacrylate, ethyl
plastic compositions with reduced gloSS by the use of methacrylate, butyl methacrylate, and the like, Styrene and
cross-linked PVC as an additive to conventional PVC siding Styrene derivatives including C.-methyl Styrene, Vinyl
and that a higher level of cross-linking will lead to lower toluene, chlorostyrene, Vinyl naphthalene, diolefins includ
gloss levels. U.S. Pat. No. 4,894,416 teaches the use of ing butadiene, isoprene, chloroprene, and the like, and
epoxide-containing resins to form additional croSS-links as a 45 mixtures of any of these types of monomers and other
means for reducing gloSS levels in ASA resins. olefinic monomers copolymerizable there with; and other
Applicants have found that the gloSS in blends comprising monomers known to those skilled in the art which will give
rigid copolymers with vinyl chloride. The amount of
PVC and ASA graft copolymers can be controlled by the comonomer that can be polymerized with vinyl chloride to
Swell indeX in the polyacrylate rubber, preferably lowering 50 give a rigid copolymer is a function of the choice of
the cross-linking indeX to within a controlled range. comonomer, as is well understood by those skilled in the art.
SUMMARY OF THE INVENTION In other embodiments wherein some of the PVC polymers
used are cross-linked, the cross-linked PVC can be chemi
The present invention relates to a method to control the cally croSS-linked, or it can be cross-linked by radiation, UV
Surface gloss of a composition of: (a) a polyvinyl chloride 55 light, heat, or post polymerization cross-linked using per
resin and (b) a graft copolymer comprising a discontinuous oxides. The term cross-linked PVC as used in this specifi
poly(alkyl(meth)acrylate) rubber phase and a rigid thermo cation is intended to include all types of cross-inked PVC,
plastic phase, wherein at least a portion of rigid thermoplas regardless of how the cross-linking is achieved.
tic phase is chemically grafted to the poly(alkyl(meth) The amount of PVC resin in the composition of the
acrylate) rubber phase, by regulating the Swell index of the 60 present invention is from 30 part by weight to about 70 parts
rubber phase in the graft copolymer to at least about 8 to by weight
vary the Surface gloSS after extrusion or molding. B. Graft Copolymer Component
In one embodiment of the present invention, the Swell The Second component of the low-gloSS composition is a
indeX is regulated by controlling the amount of the polyeth graft copolymer, comprising a discontinuous poly(alkyl
yleneically unsaturated monomer to about 0.10 to 0.40 parts 65 (meth)acrylate rubber phase and a rigid thermoplastic phase,
by weight of the polyethyleneically unsaturated monomer at least a portion of the which is chemically grafted to the
per 100 parts by weight of the alkyl(meth)acrylate monomer poly(alkyl(meth)acrylate rubber phase.
US 6,476,128 B1
3 4
Process for Preparing poly(alkyl(meth)acrylate rubber latex/ conditions. Suitable polyethylenically unsaturated mono
Substrate mers include, for example, butylene diacrylate, divinyl
The poly(alkyl(meth)acrylate rubber substrate for use in benzene, butene diol dimethacrylate, trimethylolpropane tri
making the graft copolymer in the composition is made in an (meth)acrylate, allyl methacrylate, diallyl maleate, triallyl
emulsion polymerization process. In a typical process, feed cyanurate ("TAC), triallyl isocyanurate and mixtures
Streams of reactants and reaction media, including water, a thereof.
monoethylenically unsaturated alkyl(meth) acrylate In one embodiment, the polyethylenically unsaturated
monomer, a polyethylenically unsaturated monomer, option monomer is present in amount of about 0.10 to 0.40 parts by
ally a Surfactant, and a polymerization initiator are continu weight (“pbw”) per 100 pbw of monoethylenically unsatur
ously fed to a reactor that is maintained under emulsion ated alkyl(meth)acrylate monomer.
polymerization conditions. The reactor may be heated under In one embodiment of the present invention, the polyeth
an inert atmosphere with Stirring. In one embodiment, the ylenically unsaturated monomer is triallyl cyanurate, for
reaction mixture is heated to and maintained at a reaction
used as both a cross-linking monomer and a graftlinking
OOC.
temperature from 100° F to 180° F. The poly(alkyl(meth)acrylate) rubber of the present
The product stream, in the form of an aqueous poly(alkyl 15
invention has a glass transition temperature (T) of less than
(meth)acrylate) rubber latex with an emulsion of poly(alkyl or equal to 25 C. The T is as measured by differential
(meth)acrylate) rubber particles, continuously flows from Scanning calorimetry (heating rate 20 C./minute, with the
the reactor for use in preparing the graft copolymer in the T value being determined at the inflection point). The
Step in the process. rubber particles for use in making the graft copolymer of the
In one embodiment, the monoethylenically unsaturated present invention have a weight average particle Size of 50
alkyl(meth)acrylate monomer is selected from (C-C) to 800 nm as measured by light transmission. In one
alkyl(meth)acrylate monomers and mixtures thereof, more embodiment, the index of cross-link density of the rubber as
preferably from (C-C)alkyl acrylate monomers and mix measured by the Swollen gel pulse NMR method is in the
tures thereof. AS used herein, the terminology "monoethyl range of about 20 to 50.
enically unsaturated” means having a single site of ethylenic 25 Minor amounts, Such as, for example, up to about 25 pbw
unsaturation per molecule and the terminology "(meth) per 100 pbw of the total amount monomers, of other
acrylate monomers' referS collectively to acrylate mono unsaturated monomers that are copolymerizable with the
merS and methacrylate monomers and the terminology alkyl(meth)acrylate monomer, may optionally be included
“(C-C)", as applied to a particular unit, such as, for in the reaction mixture. Suitable copolymerizable monomers
example, a chemical compound or a chemical Substituent include, for example, monoethylenically unsaturated car
group, means having a carbon atom content of from X carbon boxylic acids Such as acrylic acid, methacrylic acid, itaconic
atoms to y carbon atoms per Such unit, for example, acid, glycidyl(meth)acrylate, etc.; hydroxy(C-C)alkyl
“(C-C)alkyl” means a straight or branched alkyl Substitu (meth)acrylate monomers Such as hydroxyethyl methacry
ent group having from 1 to 12 carbon atoms per group and late; (C-C)cycloalkyl(meth)acrylate monomerS Such as
includes, e.g., methyl, ethyl, n-butyl, Sec-butyl, t-butyl, 35 cyclohexyl methacrylate; (meth)acrylamide monomerS Such
n-propyl, iso-propyl, pentyl, hexyl, heptyl, octyl, nonyl, as acrylamide and methacrylamide; maleimide monomers
decyl, undecyl and dodecyl. Suitable (C-C)alkyl(meth) Such as N-alkyl maleimides, N-aryl maleimides, maleic
acrylate monomers include (C-C) alkyl acrylate anhydride; Vinyl esterS Such as Vinyl acetate and Vinyl
monomers, e.g., ethyl acrylate, butyl acrylate, iso-pentyl propionate. Also Suitable are vinyl aromatic monomerS Such
acrylate, n-hexyl acrylate, 2-ethylhexyl acrylate, and their 40 as for example, Styrene and Substituted Styrenes having one
(C-C)alkyl methacrylate analogs Such as, e.g., methyl or more alkyl, alkoxyl, hydroxyl or halo Substituent group
methacrylate, ethyl methacrylate, propyl methacrylate, iso attached to the aromatic ring, including, e.g., C.-methyl
propyl methacrylate, butyl methacrylate, he Xyl Styrene, p-methyl Styrene, Vinyl toluene, Vinyl Xylene, tri
methacrylate, decyl methacrylate. In another embodiment, methylstyrene, butyl Styrene, chlorostyrene, dichorostyrene,
the alkyl(meth)acrylate monomer used in the process of the 45 bromoStyrene, p-hydroxystyrene, methoxystyrene; and
present invention is butyl acrylate monomer. Vinyl-Substituted condensed aromatic ring structures, Such
AS used herein, the terminology "polyethylenically unsat as, e.g., vinyl naphthalene, Vinyl anthracene, as well as
urated” means having two or more sites of ethylenic unsat mixtures of vinyl aromatic monomers, monoethylenically
uration per molecule. A polyethylenically unsaturated mono unsaturated nitrile monomerS Such as for example,
mer is used in the process of the present invention to provide 50 acrylonitrile, methacrylonitrile, C-chloro acrylonitrile.
cross-linking of the poly(alkyl(meth)acrylate) rubber par Optional Surfactants for use include one or more com
ticles formed in the proceSS and to provide "graftlinking” pounds according to formula R-SOM, wherein R is alkyl
Sites in the poly(alkyl(meth)acrylate) rubber for Subsequent or alkoxyl and M is a hydrogen radical or a cation. Examples
reaction with grafting monomers. include sodium laurylsulfate (“SLS”), sodium decyl sulfate,
In one embodiment, graftlinking monomers include those 55 Sodium 2-ethylhexyl Sulfate, potassium lauryl Sulfate,
monomers having at least one site of ethylenic unsaturation ammonium lauryl Sulfate, diethanol ammonium lauryl
that have a reactivity that is Similar, under the emulsion Sulfate, and tetraethanol ammonium lauryl Sulfate. Other
polymerization, to that of the alkyl(meth)acrylate monomer optional Surfactants for use include one or more compounds
and at least one other site of ethylenic unsaturation having according to formula R-SOM, wherein R is alkyl or
a reactivity that is substantially different from that of the 60 alkoxyl and M is a hydrogen radical or a cation. Examples
monoethylenically unsaturated alkyl(meth) acrylate include Sodium eicosyl Sulfonate and Sodium paraffin Sul
monomer, So that at least one unsaturated Site per molecule fonate. Optional Surfactants may be present in amount of
of graftlinking monomer reacts during Synthesis of the about 1 to 4 pbw of the surfactant per 100 pbw of the
rubber latex and at least one other unsaturated Site per monoethylenically unsaturated alkyl(meth)acrylate mono
molecule of graftlinking monomer remains unreacted fol 65 C.
lowing Synthesis of the rubber latex and thus remains In one embodiment, one or more initiators may be used in
available for Subsequent reaction under different reaction an amount of about 0.01 to 2 pbw. They are selected from
US 6,476,128 B1
S 6
a conventional free radical initiatorS Such as an organic plasticizers, flow promoters and other processing aids,
peroxide compound, for example, benzoyl peroxide, a per Stabilizers, colorants, mold release agents, flame retardants,
Sulfate compound Such as potassium perSulfate; an azonitrile UV Screening agents, and the like.
compound Such S 2,2'-azobis-2,3,3- Preparation of the Composition
trimethylbutyronitrile,or a redox initiator System, Such as a The production of the composition of the invention is
combination of a peroxide or hydroperoxide, Such as for done by any of the operations known for the blending and
example, hydrogen peroxide, cumene hydroperoxide extrusion of compositions containing PVC, Such as blending
(“CHP) or t-butyl hydroperoxide; an oxidizing agent such in a two-roll mill, a Banbury mixer, a single Screw extruder
as ferrous Sulfate; a chelating agent Such as, for example, or twin-Screw extruder.
tetrasodium pyrophosphate (“TSPP”), ethylene diamine tet The composition of the present invention can be formed
raacetic acid ("EDTA) or a salt of ethylene diamine tet into useful articles by a variety of known processes Such as,
raacetic acid, and a reducing agent, Such as, for example, for example, profile extrusion, sheet eXtrusion, extrusion
Sodium formaldehyde Sulfoxylate or a reducing Sugar. blow molding and thermoforming, and injection molding.
In one embodiment, about 0.01 to 1.0 pbw of an electro The composition of the present invention is particularly
lyte per 100 pbw alkyl(meth)acrylate monomer is also used. 15 well Suited as construction materials. Such as Vinyl siding
Suitable electrolytes include, for example, tetrasodium pyro applications and articles Such as, e.g., fencing, decking
phosphate and Sodium Sulfate. planks, outdoor furniture, etc.
Process for Preparing the Graft Copolymer
In the Second phase for making the graft copolymer, EXAMPLES
monomers are polymerized in the presence of poly(alkyl The process of the invention is illustrated by the following
(meth)acrylate)rubber particles made in the first phase to examples, which are not, however, to be construed as
thereby form a graft copolymer having a dispersed poly limiting in anyway.
(alkyl(meth)acrylate) rubber phase and a rigid thermoplastic In all examples, the composition is a blend of acrylate
phase, at least a portion of which is chemically grafted to the styrene-acrylonitrile (ASA) and polyvinyl vinyl chloride
poly(alkyl(meth)acrylate) rubber phase. 25
resin with a K value of about 67.
In one embodiment, the rigid thermoplastic resin phase
comprises thermoplastic polymer or copolymer that exhibits In examples 1-10, the ASA is prepared as follows for a
a T, of greater than 25°C. In another embodiment, the rigid broad size distribution of cross-linked butylacrylate rubber.
thermoplastic phase comprises a polymer having repeating The amounts of butyl acrylates and the croSS-linking agent
units derived from one or more monomerS Selected from the (s) vary depending on the examples. Example 11 employs an
group consisting of (C-C)alkyl(meth)acrylate monomers, ASA graft copolymer commercially produced under the
trade name GELOY GY 1030G ASA from General Electric
Vinyl aromatic monomers and monoethylenically unsatur Company and prepared according to U.S. Pat. No. 3,944,631
ated nitrile monomers. Suitable (C-C) alkyl(meth) with the use of divinyl benzene (“DVB") as an additional
acrylate monomers, Vinyl aromatic monomers and monoet croSS-linking monomer, at a croSS-link level of about 0.1
hylenically unsaturated nitrile monomers are those Set forth 35
pbw of ASA.
above in the description of the rubber phase. In one
embodiment, the rigid thermoplastic resin phase comprises In examples 1-10, the following monomer mixture is fed
a vinyl aromatic polymer having first repeating units derived to a stirred reaction vessel at 140 F. at a rate of 1 reactor
from one or more vinyl aromatic monomers and having volume every 90 minutes. butyl acrylate, with “pbw” indi
Second repeating units derived from one or more monoeth 40 cates part per hundred based on the weight of butyl acrylate:
ylenically unsaturated nitrile monomers. a) butyl acrylate
The rigid thermoplastic phase is made according to b) TAC (triallylcyanurate)
known processes, e.g., mass polymerization, emulsion c) 0.12 pbw CHP
polymerization, Suspension polymerization or combinations d) 0.15 TSPP
thereof, wherein a at least a portion of the rigid thermoplas 45
e) 0.05 FE
tic phase is chemically bonded, i.e., “grafted” to the rubber
phase via reaction with unsaturated SiteS present in the f) 0.25 EDTA Na2
rubber phase. The unsaturated Sites in the rubber phase are g) 0.132 SFS
provided, e.g., by residual unsaturated Sites in those repeat h) 250 demineralized water
ing units of the rubber that were derived from a graftlinking 50 In the next Step, lateX containing 32 parts of the acrylic
OOC. rubber as prepared above is charged along with 190 parts of
The amount of grafting that takes place between the rigid demineralized water to a Semi-batch emulsion polymeriza
thermoplastic phase and the rubber phase varies with the tion reactor and heated to 140 F. The acrylic rubber
relative amount and composition of the rubber phase. In one Substrate is then reacted with the following monomers and
embodiment, about 10 to 90 wt % of the rigid thermoplastic 55 additives (in pbw relative to 32 pbw of acrylic rubber, for a
phase is chemically grafted to the rubber. In a Second total of 100 parts) over 120 minutes feed time:
embodiment, about 40 to 90 wt % of the rigid thermoplastic i) 50.5 Styrene
phase remains “free,” that is, non-grafted. j) 17.5 Acrylonitrile
The amount of graft copolymer in the composition of the k) 0.275 CHP
present invention is from 30 to about 70 parts by weight. In 60
one embodiment of the invention, the graft copolymer is 1) 0.165 EDTA-Na2
acrylate-styrene-acrylonitrile (ASA). m) 0.0033 FeSO4
Optional Components n) 0-0.1 tert-dodecyl mercaptam (t-DDM) depending on
The blends of the invention may be further modified by the example
addition of other types of additives known to the art of 65 o) 0-0.1 DVB depending on the example
plastics compounding, including fillers (clay, talc, etc.), The resulting ASA graft copolymer is isolated via coagul
reinforcing agents (glass fibers), impact modifiers, lation with CaCl2 and dried in a fluidized bed dryer. The
US 6,476,128 B1
7 8
ASA graft copolymer powder is then mixed with the PVC grafted ASA resin powders are measured directly. The melt
and other additives according to the formula: flow rate (“MFR”) is measured according to ASTM D1238
a) 50 parts PVC resin (K=69) under conditions of 220 degrees C. and an applied mass of
b) 1.8 parts tin stabilizer 10 Kg.
c) 50 parts ASA resin (examples 1-10) In the examples, emulsion polymerized ASA rubbers are
characterized with respect to: a) the amount of cross-linking
d) 1 part acrylic process aid agents present as part per hundred based on the weight of
e) 0.2 parts oxidized polyethylene wax butyl acrylate, Styrene and acrylonitrile monomer charges
f) 5 parts TiO2. (ASA); b) cross-link index by pulse NMR; c) gel content (as
The blend is mixed in a high speed Henschel mixer, then % insoluble); d) swell index in acetone; e) MFR, and f)
co-extruded on a laboratory twin-Screw Siding extrusion line Siding gloSS.
ASA
Characterization
Acetone
pbw pbw pbw NMR Swell Acetone Siding
Example TAC tDDM DVB Crosslink index Index %. Insol MFR Gloss
O.15 O.O O.O 62.5 12.2 50.7 2.4 17.0
O.15 O.O O.O 618 50.5 1.9 15.8
O.15 O.O O.1 63.6 8.2 61.1 O.3 20.9
O.47 O.O O.O 70.1 7.2 58.1 18 46.8
O.47 O.O O.O 70.4 54.O 1.1 42.3
O.47 O.O O.1 71.6 6.5 61.9 O.8 48.5
O.15 O1 O.O 58.2 10.9 41.6 14.7 14.2
O.15 O1 O.1 64.1 56.4 3.0 18.3
O.47 O1 O.O 67.9 6.5 42.7 8.3 38.5
O.47 O1 O.1 70.5 5.9 55.6 2.5 49.9
GY1O3O 52.8 65.O 4.6 30.3
through a conventional dual-manifold Siding die, then onto 35
FIGS. 1 and 2 are plots of the cross-link index and Swell
a conventional Side Substrate formulation for Vinyl Sidings. indeX as a function of the Siding gloSS. It is noted that if the
Color concentrates typical for Vinyl Sidings are introduced to Swell-index goes above 20, it may have an adverse effect on
the ASA/PVC blend for the desired colors. The extrudate is impact Strength.
passed through a conventional vinyl Siding embossing roller What is claimed is:
and forming table. The feed ratios of ASA/PVC capstock 40
1. A method of controlling the Surface gloSS of an extrud
formulation and Substrate formulation are adjusted to main able composition of: (a) 30-70 parts by weight of a poly
tain an ASA/PVC capstock thickness of 0.004-0.008" and a vinyl chloride polymer and (b) a graft copolymer comprising
Substrate thickness of about 0.035". a discontinuous poly(alkyl(meth)acrylate) rubber phase hav
Weight percent solids are determined utilizing a CEM ing a weight average particle size of 50 to 800 nanometers
Labwave 9000 gravimetric microwave drier, drying to a 45
and a rigid thermoplastic phase wherein at least about 10
constant weight at 50% full microwave output. weight 9% to about 90 weight % of rigid thermoplastic phase
Gel content is determined by diluting about 0.5 g of a is chemically grafted to the poly(alkyl(meth)acrylate) rubber
Specified rubber Substrate or graft Sample into a known phase, the graft copolymer having a glass transition tem
amount of acetone (about 30 g) with Shaking at ambient perature of 25 C. or less,
temperature for 24 hours, and then centrifuging the dilute 50 said method comprises the Steps of (1) extruding the
dispersion for 1.5 hours at 15,000 rpm in a Sorvall Super composition, and (2) regulating the Swell index of the
speed RC2-B Automatic Refrigerated Centrifuge. The rubber phase in component (b) to at least about 8 to
Supernatant is removed and evaporated to dryneSS, allowing about 20 by controlling the amount of a polyethyleni
gravimetric determination of the Soluble portion to deter cally unsaturated monomer in an amount of about 0.10
mine gel content. The Substrate Swollen gel weight is 55 to 0.40 parts by weight per 100 parts by weight of
determined gravimetrically, then the Solvent contained is alkyl(meth)acrylate monomer in an emulsion polymer
carefully removed and the weight of dry insoluble gel ization reaction preparing the poly(alkyl(meth)
determined gravimetrically. The weight of Swollen gel is acrylate) rubber phase in order to vary the Surface gloss
divided by the weight of dried insoluble gel to yield the after extrusion in inverse proportion to the Swell indeX.
Swell index. 60 2. The method according to claim 1, wherein the graft
The index of cross-link density of the acrylic rubber is copolymer (b) is an acrylate-styrene-acrylonitrile resin.
measured by the pulse NMR spectroscopy using a Bruker 3. The method according to claim 1, wherein the rigid
Minispec PC120. The cross-linking index is the percentage thermoplastic phase in the graft copolymer (b) comprises a
of the NMR free induction decay signal which remains after polymer having repeating units derived from one or more
5 milliseconds delay following an initial excitation of a 90 65 monomers selected from the group consisting of (C-C)
degree pulse. The ungrafted rubber Substrates are measured alkyl(meth)acrylate monomers, vinyl aromatic monomers
as a Swollen gel in a Solution of tetrachloroethylene; the and monoethylenically unsaturated nitrile monomers.
US 6,476,128 B1
9 10
4. The method according to claim 3, wherein the rigid 8. The process of claim 5, wherein the optional Surfactant
thermoplastic phase comprises a Styrene-acrylonitrile is Selected from the group consisting of Sodium lauryl
copolymer, a C.-methyl Styrene-acrylonitrile copolymer or a Sulfate, Sodium decyl Sulfate, Sodium 2-ethylhexyl Sulfate,
mixture thereof. potassium lauryl Sulfate, ammonium lauryl Sulfate, dietha
5. The method of claim 1, wherein the graft copolymer (b) nol ammonium lauryl Sulfate, tetraethanol ammonium lauryl
is prepared by: Sulfate, Sodium eicosyl Sulfonate and Sodium paraffin Sul
fonate and mixtures thereof.
a. polymerizing in an emulsion polymerization reactor in 9. The process of claim 8, wherein the surfactant is
an aqueous medium, an alkyl(meth)acrylate monomer, Sodium lauryl Sulfate, Sodium paraffin Sulfonate or a mixture
a polyethyleneically unsaturated monomer, a polymer thereof.
ization initiator and an optional Surfactant, to obtain an 10. The process of claim 5, wherein the initiator com
aqueous product containing a poly(alkyl(meth) prises a redox initiator System, Said redox initiator System
acrylate) rubber latex, and comprising cumene hydroperoxide and Sodium formalde
b. polymerizing one or more monomerS Selected from the hyde sulfoxylate.
group consisting of (C-C)alkyl(meth) acrylate 15
11. The method according to claim 1, wherein Said
monomers, Vinyl aromatic monomers and monoethyl extrusion composition includes one or more additional
enically unsaturated nitrile monomers, with Said poly ingredients Selected from among plasticizers, flame retar
(alkyl(meth)acrylate) rubber latex. dant agents, Stabilizers, antioxidants mold release agents,
6. The method of claim 5, wherein said alkyl(meth) coloring agents, processing aids, mineral fillers, and glass
acrylate monomer is butyl acrylate. reinforcing agents.
7. The method of claim 5, wherein said polyethyleneically
unsaturated monomer comprises triallyl cyanurate.
You might also like
- Must To Know in Clinical Chemistry (From CC by Rodriguez) Quality ControlDocument59 pagesMust To Know in Clinical Chemistry (From CC by Rodriguez) Quality ControlGarth Briones100% (2)
- Us 4058649Document6 pagesUs 4058649yigitilgazNo ratings yet
- Aluminum CorrosionDocument1 pageAluminum CorrosionthanhnguyenhhvnNo ratings yet
- Topic 2 - Cells: Previous IB Exam Essay Questions: Unit 2Document4 pagesTopic 2 - Cells: Previous IB Exam Essay Questions: Unit 2ROWENA100% (2)
- Assay of Aspirin Tablets PDFDocument14 pagesAssay of Aspirin Tablets PDFamitNo ratings yet
- Polyester Resin, Resin CompositionDocument11 pagesPolyester Resin, Resin Compositionalfi alfathanaNo ratings yet
- Overview of Water-Based PaintDocument27 pagesOverview of Water-Based PaintHà Phương Nguyễn100% (4)
- Leaching and AdsorptionDocument18 pagesLeaching and AdsorptionVera Yulia Rachmawaty100% (1)
- Ethylene-Vinyl Acetate - An Overview - ScienceDirect TopicsDocument9 pagesEthylene-Vinyl Acetate - An Overview - ScienceDirect TopicsArun JoyNo ratings yet
- Cooling Tower Performance AmnsDocument35 pagesCooling Tower Performance AmnsNIHAR BEHERANo ratings yet
- Transport in PlantsDocument21 pagesTransport in Plantsapi-309893409100% (1)
- Chemical Analysis of Nickel, Cobalt, and High-Temperature AlloysDocument38 pagesChemical Analysis of Nickel, Cobalt, and High-Temperature AlloysLuigi HernándezNo ratings yet
- Expt 12 - 19Document74 pagesExpt 12 - 19sizexxl100% (1)
- The Molisch TestDocument12 pagesThe Molisch Testkamaksi100% (1)
- United States Patent: (75) Inventors: Guy Joseph Clamen, Opio (FR)Document5 pagesUnited States Patent: (75) Inventors: Guy Joseph Clamen, Opio (FR)Long An DoNo ratings yet
- United States Patent: (10) Patent No.: (45) Date of PatentDocument4 pagesUnited States Patent: (10) Patent No.: (45) Date of PatentAPEX SONNo ratings yet
- Patent Application Publication (10) Pub. No.: US 2006/0230987 A1Document8 pagesPatent Application Publication (10) Pub. No.: US 2006/0230987 A1marcela walterosNo ratings yet
- SBR LetixDocument6 pagesSBR LetixzaighumNo ratings yet
- United States Patent (19) : EaringDocument7 pagesUnited States Patent (19) : EaringVansala GanesanNo ratings yet
- Preparation of High Solids, Low Viscosity Carbonless Paper Gelatin Base McrocapsulesDocument5 pagesPreparation of High Solids, Low Viscosity Carbonless Paper Gelatin Base McrocapsulesSintong Leonardo SitungkirNo ratings yet
- US20090030151A1Document12 pagesUS20090030151A1Xiaofeng MengNo ratings yet
- ?nite States Tent n91: AssigneeDocument3 pages?nite States Tent n91: AssigneeererNo ratings yet
- Patent Application Publication (10) Pub. No.: US 2008/0086950 A1Document13 pagesPatent Application Publication (10) Pub. No.: US 2008/0086950 A1rat0708No ratings yet
- Patent For Plastic Roof TileDocument5 pagesPatent For Plastic Roof Tilepractice rosNo ratings yet
- United States Patent: Kim-Habermehl Et Al. (10) Patent N0.: (45) Date of PatentDocument9 pagesUnited States Patent: Kim-Habermehl Et Al. (10) Patent N0.: (45) Date of PatentJoseph ChenNo ratings yet
- Us7081297 PDFDocument11 pagesUs7081297 PDFrevider451No ratings yet
- US4251403Document4 pagesUS4251403Mahmudul HasanNo ratings yet
- July 28, 1964 B, Rosen 3,142,598: Method of Making Resin-Empregnated Glass Fiber Automobile Leaf SpringsDocument4 pagesJuly 28, 1964 B, Rosen 3,142,598: Method of Making Resin-Empregnated Glass Fiber Automobile Leaf SpringsffontanaNo ratings yet
- United States Patent (191 (111 4,379,197: Cipriani Et A1. (45) Apr. 5, 1983Document3 pagesUnited States Patent (191 (111 4,379,197: Cipriani Et A1. (45) Apr. 5, 1983Siswand BIn Mohd AliNo ratings yet
- USP Solvent Based CADocument6 pagesUSP Solvent Based CAMohammadJavad FotrosNo ratings yet
- Patent Application Publication (10) Pub. No.: US 2004/0191514 A1Document5 pagesPatent Application Publication (10) Pub. No.: US 2004/0191514 A1Xiaofeng MengNo ratings yet
- Taken DeyDocument11 pagesTaken DeyabhijitNo ratings yet
- Patent Application Publication (10) Pub. No.: US 2004/0024140 A1Document11 pagesPatent Application Publication (10) Pub. No.: US 2004/0024140 A1Alexander Franco CastrillonNo ratings yet
- US20140114030A1Document9 pagesUS20140114030A1sayed nasrNo ratings yet
- US6465558 - Solvent Based Adhesive CompositionDocument9 pagesUS6465558 - Solvent Based Adhesive Compositionsubramanian.sNo ratings yet
- US20060000570Document11 pagesUS20060000570arjunanpnNo ratings yet
- Problem Set 4Document4 pagesProblem Set 4Grace Melissa ChoiNo ratings yet
- US20090162620A1Document7 pagesUS20090162620A1Dragan MarićNo ratings yet
- US20050197421A1Document7 pagesUS20050197421A1Talha RafiqueNo ratings yet
- Us20080076843a1 PDFDocument13 pagesUs20080076843a1 PDFBilen SeleshiNo ratings yet
- High Cohesion Fiber FinishesDocument9 pagesHigh Cohesion Fiber Finishesali rezaeiNo ratings yet
- EP14173974NWB1Document15 pagesEP14173974NWB1Nima FakherNo ratings yet
- D2 Us20140186554aDocument12 pagesD2 Us20140186554aDonghee ChoiNo ratings yet
- Patent Application Publication (10) Pub. No.: US 2005/0019559 A1Document18 pagesPatent Application Publication (10) Pub. No.: US 2005/0019559 A1Thuận LêNo ratings yet
- MTT655 W2 ExtrusionDocument13 pagesMTT655 W2 ExtrusionCitra Adelina SitorusNo ratings yet
- Abs PVC AlloyDocument265 pagesAbs PVC AlloysaranNo ratings yet
- 2005 PDFDocument14 pages2005 PDFBattuka BatjargalNo ratings yet
- Porcelainwasteglassslip IJAC2009Document7 pagesPorcelainwasteglassslip IJAC2009Billvaraj GopalNo ratings yet
- United States Patent: (54) Encapsulating and PottingDocument8 pagesUnited States Patent: (54) Encapsulating and PottingVansala GanesanNo ratings yet
- Acrylic Emulsion Adhesive, MethodDocument8 pagesAcrylic Emulsion Adhesive, MethodAien Nabieyla1607No ratings yet
- Pressure Drop Modeling SiC FoamsDocument9 pagesPressure Drop Modeling SiC FoamsLykaios Schultz DohrnNo ratings yet
- US20020026015A1Document11 pagesUS20020026015A1Alexander Franco CastrillonNo ratings yet
- US20100331517A1 BayerDocument7 pagesUS20100331517A1 BayerAyuNo ratings yet
- US7390358Document8 pagesUS7390358lic.escobar2018No ratings yet
- US7115696Document10 pagesUS7115696Như HồNo ratings yet
- US7297674Document7 pagesUS7297674Abdulrahman HamdanNo ratings yet
- BusaDocument10 pagesBusadewiNo ratings yet
- Kordsa Patent - US20150315410A1Document5 pagesKordsa Patent - US20150315410A1Çakıl ŞenolNo ratings yet
- United States Patent (19) : Pre-Mixed Catalyzed WinylacetateDocument6 pagesUnited States Patent (19) : Pre-Mixed Catalyzed WinylacetateSHRINIL DESAINo ratings yet
- Unsat Pol EsterDocument9 pagesUnsat Pol EsterAmr Abdelmegid abdelsalam husseinNo ratings yet
- Rohm and Haas US6300409Document7 pagesRohm and Haas US6300409HermawanRandiNo ratings yet
- US5338348Document6 pagesUS5338348Omar MorteoNo ratings yet
- United States Patent (10) Patent No.: US 6,651,730 B2: Jiang Et Al. (45) Date of Patent: Nov. 25, 2003Document10 pagesUnited States Patent (10) Patent No.: US 6,651,730 B2: Jiang Et Al. (45) Date of Patent: Nov. 25, 2003Vy PhanNo ratings yet
- United States Patent (10) Patent No.: US 6,458,856 B1: Peng Et Al. (45) Date of Patent: Oct. 1, 2002Document12 pagesUnited States Patent (10) Patent No.: US 6,458,856 B1: Peng Et Al. (45) Date of Patent: Oct. 1, 2002Luigi RussoNo ratings yet
- United States PatentDocument10 pagesUnited States PatentAlexander Franco CastrillonNo ratings yet
- Iiihihiiihiiii: United States PatentDocument8 pagesIiihihiiihiiii: United States PatentGuillermoNo ratings yet
- United States Patent (19) : Jan. 29, 1985 45) Date of PatentDocument6 pagesUnited States Patent (19) : Jan. 29, 1985 45) Date of PatentSubramanian SudanthiramoorthyNo ratings yet
- Us 4925562Document7 pagesUs 4925562César Fernando Melquiades BravoNo ratings yet
- United States Patent (10) Patent No.: US 6,809,154 B2: Lindahl Et Al. (45) Date of Patent: Oct. 26, 2004Document6 pagesUnited States Patent (10) Patent No.: US 6,809,154 B2: Lindahl Et Al. (45) Date of Patent: Oct. 26, 2004ariefNo ratings yet
- Low Temp CuringDocument8 pagesLow Temp CuringsuksesNo ratings yet
- A New Coated CatalystDocument10 pagesA New Coated CatalystDhanashree JagtapNo ratings yet
- Advances in Solid Oxide Fuel Cells and Electronic Ceramics IIFrom EverandAdvances in Solid Oxide Fuel Cells and Electronic Ceramics IIMihails KusnezoffNo ratings yet
- List of Persistent and Non Persistent CargoDocument33 pagesList of Persistent and Non Persistent CargoJeet Singh100% (1)
- Chapter 15Document53 pagesChapter 15HEMANT RAMJINo ratings yet
- This Study Resource Was: Chemistry Test AssignmentDocument7 pagesThis Study Resource Was: Chemistry Test AssignmentDimson DennisNo ratings yet
- EagleBurgmann MG1 enDocument4 pagesEagleBurgmann MG1 ensanjeevvangeNo ratings yet
- Carbon Fibers From Polymer Precursor SystemsDocument174 pagesCarbon Fibers From Polymer Precursor SystemsRasha Samir SryoNo ratings yet
- This Study Resource Was: Chemical ReactionsDocument4 pagesThis Study Resource Was: Chemical ReactionsRemar Jhon PaineNo ratings yet
- DPP 1 Acidic Basic Strength VKP Sir 3688Document1 pageDPP 1 Acidic Basic Strength VKP Sir 3688ʚAwais MirzaʚNo ratings yet
- TOUCHLESS VEHICLE WASH W NATSURF 265 HCAC20v1Document1 pageTOUCHLESS VEHICLE WASH W NATSURF 265 HCAC20v1rezaNo ratings yet
- Whirlfloc TDocument2 pagesWhirlfloc TSatish AgnihotriNo ratings yet
- 20384-Polymers Information and ActivityDocument164 pages20384-Polymers Information and ActivityAbdodada DadaNo ratings yet
- Kognity Notes Bio B2Document20 pagesKognity Notes Bio B2eybasco.2001No ratings yet
- Proteins: Their Biological Functions and Primary StructureDocument59 pagesProteins: Their Biological Functions and Primary StructureIsha BhartiNo ratings yet
- Chapter 7Document3 pagesChapter 7Misbah Sajid ChaudhryNo ratings yet
- Protein TargetingDocument10 pagesProtein TargetingdwigusmalawatiNo ratings yet
- SPVC Online Test Series Pharmaceutics-Ii Module 5 Question & AnswersDocument9 pagesSPVC Online Test Series Pharmaceutics-Ii Module 5 Question & AnswersAkhil SharmaNo ratings yet
- Kobo NonD5 DispersionsDocument2 pagesKobo NonD5 DispersionswanyiwanNo ratings yet
- CO-ORDINATED SCIENCES 0654/12 Paper 1 Multiple Choice (Core)Document20 pagesCO-ORDINATED SCIENCES 0654/12 Paper 1 Multiple Choice (Core)José Antonio Álvarez CuberoNo ratings yet
- Titanium Dioxide: 4876 Tioconazole / Official Monographs USP 35Document2 pagesTitanium Dioxide: 4876 Tioconazole / Official Monographs USP 35limiyantoNo ratings yet