Professional Documents
Culture Documents
CH-1 (Xii)
Uploaded by
Vijay KumarOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CH-1 (Xii)
Uploaded by
Vijay KumarCopyright:
Available Formats
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.
) Ph - 7042177073
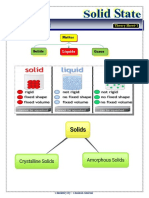
Solid are substances having definite shape and definite volume. In solids, the particles are closely packed and
the force of attraction between the particles is strong. So solids are rigid. Their constituent particles (atoms,
molecules or ions) have fixed positions and can only vibrate about their mean positions.
CLASSIFICATION OF SOLIDS
On the basis of orderly arrangement of particles, solids can be classified into two categories – Crystalline solids
and Amorphous solids.
1. Crystalline solids - In these solids, the constituent particles have a well ordered arrangement
throughout the solid, i.e., they have a long range order. They consist of a large number of small crystals.
They have a definite geometrical shape, melting point and heat of fusion. e.g.: Quartz, Diamond,
Graphite, fullerene, NaCl, CuSO4.5H2O, ice, naphthalene, SiC etc.
2. Amorphous solids - In these solids, the ordered arrangement of constituent particles is only at some
portions of the solid, i.e., they have only a short range order. The structure of these solids is similar to
that of liquids. They have no definite geometrical shape, melting point and heat of fusion. e.g.: Plastic,
Glass (quartz glass), Rubber, amorphous silica, coal, charcoal, coke, PVC etc.
Like liquids amorphous solids have a tendency to flow, though very slowly. Therefore, sometimes these
are also called pseudo solids or super cooled liquids. Glass panes fixed to windows or doors of old
buildings are slightly thicker at the bottom than at the top. This is because the glass flows down very
slowly and makes the bottom portion slightly thicker.
Amorphous solids on heating become crystalline at some temperature. Some glass objects from ancient
civilizations are found to become milky in appearance due to some crystallization.
ANISOTROPIC AND ISOTROPIC SUBSTANCES
Solids in which the physical properties like electrical conductance, refractive index etc are different when
measured in different directions are said to be anisotropic in nature. This is due to the different arrangement of
particles in different directions. Crystalline solids belong to this class.
Solids in which the physical properties are same along any direction are said to be isotropic in nature. This is
due to the irregular arrangement of particles along different directions. Amorphous solids belong to this class.
DIFFERENCES BETWEEN CRYSTALLINE SOLIDS AND AMORPHOUS SOLIDS
PROPERTIES CRYSTALLINE SOLIDS AMORPHOUS SOLIDS
Orderly arrangement of particles Long range order Short range order
Geometrical shape Definite characteritics geometrical Irregular shape
shape
Melting point Melt at sharp and characteristics Gradually soften over a range of
temperature. temperature.
Heat of fusion They have definite heat of fusion They do not have definite heat of
fusion.
Mode of Cleavage When they are cut, they split into When they are cut, they give pieces
two pieces and the newly generated with irregular surfaces.
surfaces are plain and smooth
Nature True solids Pseudo solids or super-cooled
liquids
Isotropy/Anisotropy Anisotropic in nature Isotropic in nature
THE SOLID STATE 1
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
CLASSIFICATION OF CRYSTALLINE SOLIDS
On the basis of nature of particles and binding force between the particles, crystalline solids are classified into
four types- molecular solids, ionic solids, metallic solids and covalent solids.
1. Molecular Solids - Here the constituent particles are molecules. These are further subdivided into three:
i. Non-polar molecular solids: Here the constituent particles are either atoms like Ar, He etc. Or non-
polar molecules like H2,Cl2, I2 etc and the binding force between the particles is London dispersion
forces or weak van der Waal’s forces. These are soft solids and are nonconductors of electricity. They
have low melting points and are usually liquid or gaseous state at room temperature and pressure.
ii. Polar molecular solids: Here the constituent particles are polar molecules like HCl, CO 2, SO2 etc.
and the binding force between the particles is relatively stronger dipole-dipole interactions. These are
soft and non-conductors of electricity. Their melting points are higher than those of non-polar molecular
solids. Most of them are gases or liquids at room temperature and pressure.
iii. Hydrogen bonded molecular solids: Here the constituent particles are molecules which contain
atoms like H and F, O or N. The binding force between the particles is strong hydrogen bond. They are
non-conductors of electricity and are volatile solids or soft solids at room temperature and pressure. e.g.:
H2O, NH3 etc.
2. Ionic Solids: Here the constituent particles are ions and the binding force between the particles is strong
electrostatic force of attraction (ionic bond). They are hard and brittle and have high m.p & b.p. They
are electrical insulators in the solid state, since the ions are not free to move about. But in the molten or
solution state, the ions become free to move about and they conduct electricity. e.g.: NaCl, KCl, CaCl 2
etc.
3. Metallic Solids: They contain a large number of metal ions which are surrounded by a sea of electrons.
The particles are held together by strong electrostatic force of attraction (metallic bond). Due to the
presence of a large number of free electrons, they are good conductors of heat and electricity, malleable
and ductile and show metallic lustre. e.g. All metals and alloys.
4. Covalent or Network Solids: Here the constituent particles are atoms and the binding force between
the particles is strong covalent bond. They are very strong and brittle, have extremely high melting point
and are electrical insulators. e.g. Diamond, Silicon Carbide (SiC, commonly known as Carborundum),
Quartz, Graphite etc.
Graphite has exceptional properties i.e., it is soft and good conductor of electricity. In graphite carbon
atoms are arranged in different layers and each atom is covalently bonded to three adjacent carbon
atoms. The fourth electron is free to move about between different layers. So Graphite is a good
conductor of electricity. The different layers are held together by weak van der Waal’s force of
attractions. So each layer can slide over the other and hence it is soft and used as a good lubricant.
CRYSTAL LATTICE
The regular three dimensional arrangements of constituent particles of a crystal in space is called crystal lattice
or space lattice.
The important characteristics of a crystal lattice are:
1. Each point in a lattice is called lattice point or lattice site.
2. Each point in a crystal lattice represents one constituent particle which may be an atom, a molecule
(group of atoms) or an ion.
3. Lattice points are joined by straight lines to bring out the geometry of the lattice.
(There are only 14 possible three dimensional lattices. These are called Bravais Lattices.)
UNIT CELL
A unit cell is the smallest portion of a crystal lattice which, when repeated in three dimension to generate an
entire lattice. Or, it is the building block of a crystal. A unit cell is characterised by its edge lengths (a, b and c)
THE SOLID STATE 2
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
and angle between the edges – α (between b and c), β (between a and c) and γ (between a and b). Thus a unit
cell is characterised by 6 parameters – a, b, c, α, β and γ.
Unit cells can be broadly divided into two - primitive and centred unit cells.
1. Primitive Unit Cells: Here the constituent particles are present only at the corners of the unit cell.
2. Centred Unit Cells: Here the constituent particles are present at the corners and other positions of the
unit cell.
These are of three types:
i. Body-centred unit cells: Here the constituent particles are present at the body centre and at the corners
of the unit cell.
ii. Face-centred unit cells: Here the constituent particles are present at the centre of each faces and at
the corners of the unit cell.
iii. End-centred unit cells: Here the constituent particles are present at the centre of any two opposite
faces and at the corners of the unit cell.
CALCULATION OF NUMBER OF ATOMS IN A UNIT CELL (z)
Primitive cubic (Simple Cubic) unit cell:
Here atoms are present only at the corners of the cube. Each corner atom is shared by 8 unit cells.
Therefore, contribution to one unit cell = 1/8
Since each unit cell has 8 atoms at the corners, the total number of atoms in one unit cell = 8×1/8 = 1 atom
So for a primitive (simple cubic) unit cell, z = 1
Body-centred cubic (bcc) unit cell:
Here the particles are present at the corners of the cube and also one atom at the body centre.
The number of atoms at the corner = 8×1/8 = 1
The atom present at the centre of the body is not shared by other atoms.
So the number of atoms at the body-centre = 1
Therefore, total number of atoms in the unit cell = 1+1 = 2 atoms
So, for a bcc, z = 2
Face-centred cubic (fcc) unit cell:
Here the atoms are present at the corners and also at the centre of each faces. Each corner atom is shared by 8
unit cells and each face centre atom is shared by 2 unit cells.
Number of corner atoms = 8×1/8 = 1
Number of face-centre atoms = 6×1/2 = 3
Therefore, total number of atoms = 1+3 = 4
So, for an fcc, z = 4
CLOSE PACKING IN SOLIDS
In solids the particles are closely packed. In close packed structures the particles are considered as hard spheres.
Solids are three dimensional and the 3 dimensional structure can be obtained by the following three steps:
1. Close packing in One Dimensions
Here the spheres are arranged in a row touching each other. In this arrangement each sphere is in contact
with 2 adjacent spheres. Therefore, co-ordination number of each sphere is 2.
2. Close packing in Two Dimensions
Here the spheres are arranged in two directions – length-wise and breadth-wise. This can be done in two
different ways.
i. Square close packing in two dimensions: Here the spheres of second row are placed exactly above
those of the first row. In this arrangement, each sphere is in contact with four adjacent spheres. So the
co-ordination number of each sphere is 4. When we join the centres of these spheres, we get a square.
So this close packing is called square close packing in two dimensions.
ii. Hexagonal close packing in two dimensions: Here the spheres of the second row are placed in the
depressions of the first row, the spheres of the third row are placed in the depressions of the second row
THE SOLID STATE 3
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
and so on. In this arrangement, each sphere is in contact with six adjacent spheres. So the co-ordination
number of each sphere is 6. When we join the centres of these spheres, we get a hexagon. So this close
packing is called hexagonal close packing in two dimensions.
(Hexagonal close packing is more efficient than square close packing in two dimensions. This is
because in Hexagonal close packing maximum space is occupied by spheres.)
3. Three Dimensional close packing
Here the particles are arranged in layers. This can be possible in two ways.
i. Three dimensional close packing from two dimensional square close-packed layers: Here the spheres
of the second layer are placed exactly above those of the first layer. In this arrangement spheres of both
the layers are perfectly aligned horizontally as well as vertically. The spheres of the third layer are
placed exactly above those of the second layer and so on. If the arrangement of the spheres in the first
layer is denoted as ‘A’, all the layers are of ‘A’ type. So this arrangement forms AAA….. type pattern.
The lattice thus generated is the simple cubic lattice and its unit cell is the primitive cubic unit cell.
ii. Three dimensional close packing from two dimensional hexagonal close-packed layers: Here the first
layer is arranged as hexagonal manner. The second layer is placed above the depressions of the first
layer. On placing the second layer there arises two types of voids (vacant spaces) above the second layer
– tetrahedral voids and octahedral voids. Thus when we place the third layer over the second there are
two possibilities:
Covering tetrahedral voids: Here the spheres of the third layer are placed above the tetrahedral voids of
the second layer. In this arrangement, the spheres of the third layer are vertically above those of the first
layer, i.e. the first layer and the third layer are identical. If we call the first layer as ‘A’ and the second
layer as ‘B’, then the third layer will be ‘A’, the fourth layer will be ‘B’ and so on. This will form the
pattern ABAB…… This type of close packing is called Hexagonal close packing (hcp) in three
dimensions. This type of arrangement is found in metals like Mg, Zn etc.
Covering octahedral voids: Here the spheres of the third layer are placed above the octahedral voids of
the second layer. In this arrangement, the third layer is different from the first or the second layer. But
the spheres of the fourth layer are vertically above those of the first layer, i.e. the first layer and the
fourth layer are identical. If we call the first layer as ‘A’, the second layer as ‘B’, and the third layer as
‘C’, then the fourth layer will be ‘A’, the fifth layer will be ‘B’ and so on. This will form the pattern
ABCABC…… This type of close packing is called Cubic close packing (ccp) or face-centred cubic(fcc)
packing in three dimensions. This type of arrangement is found in metals like Cu, Ag etc.
(In both hcp and ccp 74% of the available space is occupied by spheres. So both are equally efficient.)
CO-ORDINATION NUMBER
In a close packed arrangement the number of nearest neighbours with which a given sphere is in contact is
called the co-ordination number of that sphere. In both hcp and ccp each sphere is in contact with 12 adjacent
spheres. Thus the co-ordination number in both hcp and ccp is 12.
INTERSTITIAL VOIDS
The vacant space in close packed arrangement is called voids. These are of two typestetrahedral voids and
octahedral voids.
Tetrahedral void: A void surrounded by four spheres in tetrahedral position is called tetrahedral void. In a
close packed arrangement the number of tetrahedral voids is double the number of spheres, i.e. there are two
THE SOLID STATE 4
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
tetrahedral voids per sphere.
Octahedral voids: A void surrounded by six spheres in octahedral position is called octahedral void. In a close
packed arrangement the number of octahedral voids is equal to the number of spheres, i.e. there is only one
octahedral void per sphere.
If there are N close packed spheres,
The number of tetrahedral voids = 2N
The number of octahedral voids = N
PACKING EFFICIENCY
The percentage of the total space occupied by spheres (particles) is called packing efficiency.
Volume occupied by all the spheres in the unit cell
Packing Efficiency = ------------------------------------------------------------------× 100 %
Total volume of the unit cell
Calculation of Packing Efficiency
1. In simple cubic structures:
Consider a cube with edge length ‘a’ and the radius of the particle ‘r’. Here the edge length is related to
the radius of the particle a = 2r
The volume of the cubic unit cell = a3 = (2r)3 =8r3
A simple cubic unit cell contains only one particle.
Volume of one sphere = (4/3) πr3
Volume occupied by the spheres in the unit cell
Packing Efficiency = ----------------------------------------------------------- × 100 %
Total volume of the unit cell
(4/3) πr3
= -------------×100 % = π/6 × 100 % = 52.4 %
8r3
THE SOLID STATE 5
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
2. In hcp and ccp structures:
Consider a cube with edge length ‘a’ and face diagonal ‘b’
In ΔABC, AC2 = AB2+BC2
i.e. b2 = a2+a2
or, b2 = 2a2
or, b= √2a
If ‘r’ is the radius of the sphere, then b = 4r
therefore, 4r = √2a
Or, a = 4r/√2 = 2√2r
We know that, volume of a sphere = (4/3)πr3
In ccp (fcc) or hcp, there four spheres per unit cell
Volume of four spheres = 4×(4/3)πr3
Volume of the cube = a3 = (2√2r)3
Volume occupied by four spheres in the unit cell
Packing Efficiency = ----------------------------------------------------------- × 100 %
Total volume of the unit cell
4×(4/3)πr3
= -------------- × 100 %
(2√2r)3
(16/3) πr3
= --------------- × 100 % = 74 %
16√2r3
3. In Body-centred cubic (bcc) structures:
Consider a cube with edge length ‘a’, face diagonal ‘b’ and body diagonal ‘c’. From the figure it is clear
that the atom at the centre is in contact with the other two atoms diagonally placed.
THE SOLID STATE 6
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
In ΔEFD, ED2 = EF2 + ED2
i.e. b2 = a2 + a2 = 2a2
or, b = √2a
In ΔAFD, AF2 = AD2 + FD2
i.e. c2 = a2 + b2 = a2 + 2a2 = 3a2
Or, c = √3a
But, c = 4r (where r is the radius of the particle)
4r = √3a
Or, a = 4r/√3 (also r = √3a/4)
In a bcc, the no. of atoms present per unit cell = 2
Volume of 2 spheres = 2 × (4/3)πr3
Volume of the cube = a3 = (4r/√3)3
Volume occupied by two spheres in the unit cell
Packing Efficiency = ------------------------------------------------------------ × 100 %
Total volume of the unit cell
2×(4/3)πr3
= -------------- × 100 %
(4r/√3)3
(8/3) πr3
= -------------- × 100 % = 68 %
64/(3√3)r3
CALCULATION OF DENSITY OF THE UNIT CELL (SOLID)
Consider a cubic unit cell with edge length ‘a’. Then volume of the unit cell = a3.
Let ‘M’ be the atomic mass of the element in the unit cell {i.e. mass of Avogadro number (NA) of atoms}.
Then mass of one atom = M/ NA.
Let the number of particles present per unit cell = z
Then mass of the unit cell = z x M/ NA
Mass of the unit cell
Density of the unit cell = -----------------------------
Volume of the unit cell
z.M
i.e. density (d) = ------------
a3 . NA
IMPERFECTIONS IN SOLIDS
In crystalline solids, the complete orderness of constituent particles is seen only at low temperature. At normal
temperature there arise some irregularities in the orderly arrangement of particles. These irregularities are
termed as imperfections or crystal defects.
The crystal defects are broadly classified into two – point defects and line defects.
If the deviation from ideal arrangement is around a point or an atom in a crystalline substance, it is termed as
point defect. If the irregularities or deviation from ideal behaviour is in the entire rows of lattice points, it is
termed as line defect.
POINT DEFECT
Point defects can be classified into three types: Stoichiometric defects, Non-stoichiometric defects and Impurity
defects.
THE SOLID STATE 7
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
Stoichiometric defects: These are point defects which do not disturb the stoichiometry of the solid. They are
also called intrinsic or thermodynamic defects, because these defects can also develop when a substance is
heated.
1. Vacancy defect: When some of the lattice sites are vacant, the crystal is said to have vacancy defect.
This defect decreases the density of the solid.
2. Interstitial defect: When some constituent particles occupy an interstitial site, the crystal is said to have
interstitial defect. This defect increases the density of the solid.
The above two types of defects are shown by non-ionic solids.
Ionic solids show two types of stoichiometric defects – Schottky defect and Frenkel defect.
3. Schottky defect: It is basically a vacancy defect. It arises due to the missing of equal number of anions
and cations from the lattice site. It is shown by ionic crystals in which the anionic and cationic sizes are
almost equal. NaCl, KCl, CsCl, AgBr etc. show Schottky defect. Due to this defect the density of the
solid decreases.
4. Frenkel defect: It is basically an interstitial defect. It arises due to the misplacing of an ion (generally a
cation) from the lattice site to the interstitial site. It is also called dislocation defect. This type of defect
is shown by ionic solids in which there is a large difference in the size of the ions. E.g. ZnS, AgCl,
AgBr, AgI etc. This defect does not change the density of the solid.
Non-Stoichiometric defects: These are point defects which change the stoichiometry of a solid. These defects
are of two types: i) metal excess defect and ii) metal deficiency defect.
1. Metal excess Defect: Here the number of cations is greater than the number of anions. This arises in
two ways.
(i) Metal excess defect due to anionic vacancies: Here some of the anions are missing from the lattice
site. The electrical neutrality is maintained by occupying electrons in the anionic sites. These electrons
are called f-centres because they give colour to the crystal. This defect is shown by alkali metal halides.
For example when NaCl is heated in an atmosphere of sodium vapour, some sodium atoms are
deposited at the surface of the crystal. The Cl ─ ions diffuse to the surface of the crystal and combines
with Na atom to form NaCl.
Na + Cl─ → NaCl + e-
THE SOLID STATE 8
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
The electron so formed diffuse into the crystal and occupies the anion vacancy. These electrons absorb
light energy and get excited. As a result the crystal becomes yellow in colour. Similarly, excess of Li
makes LiCl crystals pink and excess of K makes KCl crystals violet.
(ii) Metal excess defect due to extra cations at interstitial sites: Here some cations occupy the
interstitial sites. The electrical neutrality is maintained by occupying some electrons in adjacent
interstitial sites. E.g. When ZnO crystals are heated, the white coloured crystals becomes yellow. This is
because on heating, the crystal loses oxygen as follows:
ZnO → Zn2+ + ½ O2 + 2e-
The Zn ions now move to the interstitial sites and the electrons to neighbouring interstitial sites.
2. Metal deficiency Defect: Here the number of cations is smaller than the number of anions. This is
mainly arises due to cation vacancies. A typical example of this type is FeO. In FeO, two out of the three
Fe2+ ions are converted to Fe3+ and the third Fe2+ is missing. This creates a vacancy in the crystal lattice.
So the molecular formula of FeO is Fe0.95O. It may actually ranges from Fe0.93O to Fe0.96O.
3. Impurity Defects: It is the defect arising due to the presence of foreign particles in a crystal. For
example if molten NaCl containing a little amount of SrCl 2 is crystallised, some of the sites of Na + ions
are occupied by Sr2+. Each Sr2+ replaces two Na+ ions. It occupies the site of one ion and the other site
remains vacant. The cationic vacancies thus produced are equal in number to that of Sr 2+ ions. Another
similar example is the solid solution of CdCl2 and AgCl.
PROPERTIES OF SOLIDS
Electrical properties: Based on the electrical conductivity, solids are classified into three types:
(i) Conductors: They are solids which allow the passage of electricity through them. Their conductivity
ranges from 104 to 107 ohm-1m-1. Metals have conductivities in the order of 107 ohm-1m-1.
(ii) Semi-conductors: They are solids which allow the passage of electricity only partially. Their
conductivity ranges from 104 to 10-6 ohm-1m-1.
(iii) Insulators: They are solids which do not allow the passage of electricity through them. Their
conductivity ranges from 10-10 to 10-20 ohm-1m-1.
Conduction of Electricity in metals - Band Model
Metals conduct electricity in solid as well as in molten state. The conductivity of metals depends upon the
number of valence electrons. The atomic orbitals of metals combine to form molecular orbitals, which are so
THE SOLID STATE 9
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
closely spaced that they form a band. If this band is partially filled or it overlaps with a higher energy
unoccupied conduction band, the electrons can flow easily under an applied electric field and the metal shows
conductivity. If the gap between filled valence band and the unoccupied conduction band is large the electrons
cannot jump to it and such substances act as insulators.
Conduction of Electricity in semi-conductors
In the case of semiconductors, the gap between the valence band and the conduction band is small. So some
electron may jump from valence band to conduction band and show some conductivity. Their conductivity
increases with rise in temperature, since more electrons can jump to the conduction band. Such semiconductors
are also called intrinsic semiconductors. E.g.: Si, Ge etc.
The conductivity of intrinsic semiconductors is very low. Their conductivity can be increased by adding an
appropriate impurity. The process is called doping. Addition of impurities creates electronic defects in them.
Such semiconductors are called extrinsic semiconductors. Doping can be done by the addition of either electron
rich impurity or electron deficit impurity.
n-type semiconductors : When a group 14 (which contains 4 electrons in the valence shell) element
like Si or Ge is doped with a group 15 element (which contains 5 electrons in the valence shell) like P or
As, four electrons are used for the formation of covalent bonds and the fifth electron becomes free. The
presence of this delocalised electron increases the conductivity and hence silicon doped with electron
rich impurities is called n-type semiconductor.
p-type semiconductors : When a group 14 (which contains 4 electrons in the valence shell) element
like Si or Ge is doped with a group 13 element (which contains 3 electrons in the valence shell) like B,
Al, or Ga, the three electrons are used for the formation of covalent bonds and the fourth valence
electron is missing. This creates an electron hole or electron vacancy. An electron from a neighbouring
atom can come and fill the electron hole. So the position of the hole is moved in the direction opposite
to that of the electron has moved. Under the influence of electric field, electrons would move towards
the positively charged plate through electronic holes. It would appear as if electron holes are positively
charged. This type of semiconductors are called p-type semiconductors.
THE SOLID STATE 10
CHEMISTRY (XI & XII) by VIJAY KUMAR (M.Sc., B.Ed.) Ph - 7042177073
[N/B - A large variety of solids which have lattices similar to Ge or Si have been prepared by the
combination of groups 13&15 or 12&16. E.g. for 13and15 group compounds are InSb, AlP & GaAs.
They are used as semiconductors. E.g. for 12 and 16 group compounds are ZnS, CdS, CdSe & HgTe.]
Magnetic properties
Every solid has some magnetic properties associated with it due to the presence of electrons. Each electron in
an atom behaves like a tiny magnet. The magnetic moment originates from the orbital motion and the spin
motion of electrons. Electron being a charged particle and due to these motions, has a permanent spin and
orbital magnetic moment. The magnitude of this magnetic moment is very small and is a measured in the unit
called Bohr Magneton (μB). {1 μB = 9.27×10-24 Am2 (ampere-metresquare)}.
Based on the magnetic properties, solids can be classified into five types-
1. Diamagnetic Substances: These are weakly repelled by a magnetic field. Diamagnetism arises due to
the presence of only paired electrons. Pairing of electrons cancels their magnetic moments and they lose
their magnetic character. They are weakly magnetised in a magnetic field in opposite direction. E.g.:
H2O, NaCl, Benzene (C6H6).
2. Paramagnetic Substances: They are weakly attracted by a magnetic field. Paramagnetism is due to the
presence of one or more unpaired electrons. They are magnetised in a magnetic field in the same
direction. They lose their magnetism in the absence of external magnetic field. Eg: O 2, Cu2+, Fe3+, Cr3+
etc.
3. Ferromagnetic Substances: They are very strongly attracted by a magnetic field and can be
permanently magnetised. In solid state, the metal ions of ferromagnetic substances are grouped together
into small regions called domains. In the absence of an external magnetic field, these domains are
randomly oriented and their magnetic moments get cancelled. When the substance is placed in a
magnetic field, all the domains get oriented in the direction of the magnetic field and a strong magnetic
effect is produced. This ordering of domains persists even when the magnetic field is removed and so
they become permanent magnets. Eg: Fe, Co, Ni, Gd (Gadolinium), CrO2 etc.
4. Anti-ferromagnetic Substances: Here the domains are oppositively oriented and cancel each other. So
they have no net magnetic moment. Eg: MnO, MnO2.
5. Ferrimagnetic Substances: Here the domains are arranged in opposite directions but in unequal
numbers. They are weakly attracted by a magnetic field and have a net magnetic moment. Eg: Fe 3O4
(magnetite) and ferrites like MgFe2O4, ZnFe2O4 etc.
[N/B - Students are advised to learn Table 1.2 & 1.3 given in NCERT book as some theoretical questions are
directly based upon them]
THE SOLID STATE 11
You might also like
- Physical ChemistryDocument5 pagesPhysical ChemistryJothi KNo ratings yet
- Chpter 1 SolidsDocument30 pagesChpter 1 SolidsRahil HassanNo ratings yet
- Solid StateDocument17 pagesSolid Statenoorunnisa0184No ratings yet
- UNIT - II InorganicDocument28 pagesUNIT - II Inorganicharirajans71No ratings yet
- The Solid State: Crystalline SolidsDocument3 pagesThe Solid State: Crystalline Solidsmillinagi95No ratings yet
- LP - GP - Coulomb's LawDocument9 pagesLP - GP - Coulomb's LawAllyza SobosoboNo ratings yet
- LP - GC - Nature of SolidsDocument9 pagesLP - GC - Nature of SolidsAllyza SobosoboNo ratings yet
- Solid StateDocument49 pagesSolid Statekishangopi123No ratings yet
- 1.solid StateDocument33 pages1.solid StateBHAVITH SD VNS 06No ratings yet
- Solid StateDocument25 pagesSolid StateDigvijay SolankiNo ratings yet
- JEE Advanced 2023 Solid State Revision Notes - Free PDF DownloadDocument11 pagesJEE Advanced 2023 Solid State Revision Notes - Free PDF Downloadhishamkalliyath19No ratings yet
- Us To Make Solids of Desired PurposeDocument5 pagesUs To Make Solids of Desired PurposeSam JonesNo ratings yet
- Chapter 3Document9 pagesChapter 3JeromeNo ratings yet
- Amorphous and Crystalline SolidsDocument8 pagesAmorphous and Crystalline SolidsAli ImranNo ratings yet
- Noo Xii Ch01 Solid StateDocument51 pagesNoo Xii Ch01 Solid StateG boiNo ratings yet
- Solidstate Part 1Document35 pagesSolidstate Part 1Gopikrishna ThirunavukarasuNo ratings yet
- 5921apni KakshaDocument109 pages5921apni KakshaVimal PrasadNo ratings yet
- Types of SolidDocument37 pagesTypes of Solidmjlngpogi.walangibaNo ratings yet
- Class 12 Chemistry Chapter 1 Solid States (Typed Notes)Document12 pagesClass 12 Chemistry Chapter 1 Solid States (Typed Notes)Shaku JoshiNo ratings yet
- Solid State Notes Jee Main and Advanced PDFDocument36 pagesSolid State Notes Jee Main and Advanced PDFVedant BiyaniNo ratings yet
- Unit 1: The Solid StateDocument30 pagesUnit 1: The Solid StateDhwani KapoorNo ratings yet
- Genchem2 q3m2b Solids-And-imf FinalDocument50 pagesGenchem2 q3m2b Solids-And-imf Finalroxtonandrada8No ratings yet
- Unit III CrystollographyDocument56 pagesUnit III Crystollographymwita mwitaNo ratings yet
- Solid State Day 1Document8 pagesSolid State Day 1Rakesh SenNo ratings yet
- Solid StateDocument11 pagesSolid StateGomathi VarshiniNo ratings yet
- Material Downloaded From - 1 / 16Document156 pagesMaterial Downloaded From - 1 / 16Pralabh AgarwalNo ratings yet
- 12 Chemistry Notes ch01 The Solid State PDFDocument16 pages12 Chemistry Notes ch01 The Solid State PDFgaurav sahuNo ratings yet
- Solid State Class 12 Notes by Furqan Ahmad SirDocument7 pagesSolid State Class 12 Notes by Furqan Ahmad SirANJANA SHARMANo ratings yet
- 1 The Solid State New (Repaired) (Repaired)Document20 pages1 The Solid State New (Repaired) (Repaired)Shesha krishnaNo ratings yet
- SheetDocument108 pagesSheetPinkyNo ratings yet
- Difference Between Crystalline and Amorphous SolidsDocument24 pagesDifference Between Crystalline and Amorphous SolidsShashwat KhuranaNo ratings yet
- The Solid StateDocument41 pagesThe Solid StateComedy BoyNo ratings yet
- 1 Solid State 25Document25 pages1 Solid State 25Aryan POONIANo ratings yet
- Solids 150702201407 Lva1 App6892Document9 pagesSolids 150702201407 Lva1 App6892Samina ZaibNo ratings yet
- Solid State Notes PDFDocument36 pagesSolid State Notes PDFGyanendra Gs100% (1)
- GEN CHEM 2 LESSON 3 Intermolecular Forces of Solids and Their Properties1.1Document27 pagesGEN CHEM 2 LESSON 3 Intermolecular Forces of Solids and Their Properties1.1Loraine Castro0% (1)
- Solid StateDocument37 pagesSolid StateSai Sasivardhan GampaNo ratings yet
- Chemistry: Class - 12 SubjectDocument7 pagesChemistry: Class - 12 SubjectHemant YadavNo ratings yet
- Intermolecular Forces of Liquids and Solids Solids and Their PropertiesDocument39 pagesIntermolecular Forces of Liquids and Solids Solids and Their PropertiesSTANNo ratings yet
- General Chemistry 2 - Q3 - SLM4Document11 pagesGeneral Chemistry 2 - Q3 - SLM4Jonnel RoqueNo ratings yet
- Namma Kalvi 12th Chemistry Unit 6 PPT Material EM 219395Document93 pagesNamma Kalvi 12th Chemistry Unit 6 PPT Material EM 219395Anant Mathew SibyNo ratings yet
- ES Module 3 - Quarter 1 - Types of SolidsDocument13 pagesES Module 3 - Quarter 1 - Types of SolidsAnalynAsuncionAtaydeNo ratings yet
- 12th Science HSC Chemistry I PDFDocument14 pages12th Science HSC Chemistry I PDFSayyed SohelNo ratings yet
- Rizal General Chemistry 2 q3 Slm4Document12 pagesRizal General Chemistry 2 q3 Slm4Darlene OpeñaNo ratings yet
- 12 Chemistry Notes Ch01 The Solid StateDocument16 pages12 Chemistry Notes Ch01 The Solid Statehimanshu kumarNo ratings yet
- Solid State Chemistry Maharashtra State BoardDocument27 pagesSolid State Chemistry Maharashtra State BoardPankaj JindamNo ratings yet
- Intermolecular Forces of Liquids and Solids Solids and Their Properties PDFDocument13 pagesIntermolecular Forces of Liquids and Solids Solids and Their Properties PDFpieNo ratings yet
- Crystalline Solids: 4. Clean Cleavage With KnifeDocument5 pagesCrystalline Solids: 4. Clean Cleavage With KnifeShin Se KyungNo ratings yet
- Intermolecular Forces of Liquids and Solids Solids and Their PropertiesDocument59 pagesIntermolecular Forces of Liquids and Solids Solids and Their PropertiesAndre Jose ErminoNo ratings yet
- CHAPTER-solid State Part 1Document2 pagesCHAPTER-solid State Part 1Arpandeep KaurNo ratings yet
- Solid:-A Solid Is Defined As The Substances Which Possess Rigidity Have ADocument2 pagesSolid:-A Solid Is Defined As The Substances Which Possess Rigidity Have ASaatwat CoolNo ratings yet
- CBSE NCERT Solutions For Class 12 Chemistry Chapter 1: Back of Chapter QuestionsDocument32 pagesCBSE NCERT Solutions For Class 12 Chemistry Chapter 1: Back of Chapter QuestionsSIMPINo ratings yet
- Solid StateDocument166 pagesSolid StateSanghamitra ChakrabortyNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (The Solid State) Topic: Solids and Their ClassificationDocument4 pagesCbse Test Paper-01 CLASS - XII CHEMISTRY (The Solid State) Topic: Solids and Their ClassificationAtashImranNo ratings yet
- Matter Mol Scale Part 2Document25 pagesMatter Mol Scale Part 2naomikarenbellNo ratings yet
- Class 12 Chemistry Revision Notes The Solid StateDocument21 pagesClass 12 Chemistry Revision Notes The Solid StateAfreen AnzNo ratings yet
- SolidsDocument2 pagesSolidsNimz StudioNo ratings yet
- Solid StateDocument16 pagesSolid StateAman DeepNo ratings yet
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- Children Encyclopedia Chemistry: The World of KnowledgeFrom EverandChildren Encyclopedia Chemistry: The World of KnowledgeRating: 5 out of 5 stars5/5 (3)
- Stoichiometry - 1: Concept of Gram AtomDocument36 pagesStoichiometry - 1: Concept of Gram AtomVijay KumarNo ratings yet
- Chemical Kinetics: Rate of A ReactionDocument49 pagesChemical Kinetics: Rate of A ReactionVijay KumarNo ratings yet
- CHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Document10 pagesCHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Vijay KumarNo ratings yet
- CHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Document8 pagesCHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Vijay Kumar100% (1)
- IUPAC Nomenclature of Organic Chemistry: Basic PrinciplesDocument13 pagesIUPAC Nomenclature of Organic Chemistry: Basic PrinciplesVijay KumarNo ratings yet
- BKLT 1206-1Document6 pagesBKLT 1206-1Vijay KumarNo ratings yet
- BKLT 1202Document10 pagesBKLT 1202Vijay KumarNo ratings yet
- Coordination Compounds: Chapter - 9Document9 pagesCoordination Compounds: Chapter - 9Vijay KumarNo ratings yet
- The Transition Elements (D-Block)Document8 pagesThe Transition Elements (D-Block)Vijay KumarNo ratings yet
- Imperfections in Solid Materials - Ch4Document40 pagesImperfections in Solid Materials - Ch4aa454No ratings yet
- Tungsten Carbides: Alexey S. Kurlov Aleksandr I. GusevDocument253 pagesTungsten Carbides: Alexey S. Kurlov Aleksandr I. GusevHOSSIEN100% (1)
- Solid StateDocument219 pagesSolid StateBrimstoneNo ratings yet
- And Structure Determination: Crystal GeometryDocument22 pagesAnd Structure Determination: Crystal GeometryabhinavNo ratings yet
- CH 2Document19 pagesCH 2Abdul RashidNo ratings yet
- Basics of X-Ray DiffractionDocument13 pagesBasics of X-Ray Diffractionleizar_death64No ratings yet
- Fearful Symmetry... Is God A Geometer - Ian Stewart, Martin Golubitsky - (Penguin Press - 1992 - Pp.310)Document310 pagesFearful Symmetry... Is God A Geometer - Ian Stewart, Martin Golubitsky - (Penguin Press - 1992 - Pp.310)Håkan Strömberg100% (1)
- Metallurgy Module One Notes CompleteDocument45 pagesMetallurgy Module One Notes CompleteMutai Daniel50% (2)
- AA Metatron The Alchemy of Crystalline Phi Merlin's WandDocument15 pagesAA Metatron The Alchemy of Crystalline Phi Merlin's WandMeaghan MathewsNo ratings yet
- EP104 Sen LNT 003a Crystal Structure May11Document60 pagesEP104 Sen LNT 003a Crystal Structure May11Walid AliNo ratings yet
- ME2151 Tutorial Solution 1Document21 pagesME2151 Tutorial Solution 1benjaminyc96No ratings yet
- Mitzi 2001Document12 pagesMitzi 2001Reena BalharaNo ratings yet
- Semiconductor Fundamentals-2ed R. F. Pierret Prentice Hall 1988 PDFDocument154 pagesSemiconductor Fundamentals-2ed R. F. Pierret Prentice Hall 1988 PDFMina SamehNo ratings yet
- Mse215 Full-BirleştirildiDocument352 pagesMse215 Full-BirleştirildiyagmurdeniiizzzNo ratings yet
- An Introduction To Crystallography and Mineral Crystal SystemsDocument30 pagesAn Introduction To Crystallography and Mineral Crystal SystemsLuis Paez80% (5)
- Crystallography: Branch of Physics Which Deals With The Arrangement of Atoms or Molecules in MatterDocument19 pagesCrystallography: Branch of Physics Which Deals With The Arrangement of Atoms or Molecules in MatterNirban SahaNo ratings yet
- Synthesis of Nanostructured Materials by Mechanical Milling: Problems and OpportunitiesDocument10 pagesSynthesis of Nanostructured Materials by Mechanical Milling: Problems and OpportunitiesGuillermo IdarragaNo ratings yet
- APpNotes - Temperature Coefficient of Resistance For Current SensingDocument5 pagesAPpNotes - Temperature Coefficient of Resistance For Current SensingMeral MeralNo ratings yet
- Defects in SolidsDocument47 pagesDefects in SolidsHarsha IndurtiNo ratings yet
- Thermal Conductivity: Theory, Properties, and ApplicationsDocument305 pagesThermal Conductivity: Theory, Properties, and Applicationsxiao seanNo ratings yet
- Crystal Directions and PlanesDocument16 pagesCrystal Directions and PlanesSameh AhmedNo ratings yet
- Self-Learning Module in General Chemistry Ii Lesson:: Quarter: 3 Week: 2 Day and TimeDocument16 pagesSelf-Learning Module in General Chemistry Ii Lesson:: Quarter: 3 Week: 2 Day and TimeCess BagtasNo ratings yet
- The Optical TransformDocument6 pagesThe Optical TransformJoan MNo ratings yet
- Final MSTDocument41 pagesFinal MSTPrakhar ParasharNo ratings yet
- HW 03 S1 2324 Group NumberDocument12 pagesHW 03 S1 2324 Group NumberNhư TâmNo ratings yet
- Biomaterials, Medical Devices and Tissue Engineering An Integrated Approach (Frederick H. Silver PHD (Auth.) )Document309 pagesBiomaterials, Medical Devices and Tissue Engineering An Integrated Approach (Frederick H. Silver PHD (Auth.) )luis miguel ballesteros ospinaNo ratings yet
- Chemistry 1st Year Test (4) 1Document2 pagesChemistry 1st Year Test (4) 1Rashid JalalNo ratings yet
- Chapter 2 Defect AssociationsDocument7 pagesChapter 2 Defect AssociationsNesh N. López MeléndezNo ratings yet
- Introduction To Materials and Processes PDFDocument0 pagesIntroduction To Materials and Processes PDFjayeshjpillaiNo ratings yet
- Chapter - 03 - 2-Crystal StructuresDocument24 pagesChapter - 03 - 2-Crystal Structuresyelten14No ratings yet