Professional Documents
Culture Documents
1 Solid State 25
Uploaded by
Aryan POONIAOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1 Solid State 25
Uploaded by
Aryan POONIACopyright:
Available Formats
Solid State, Unit-1
Solid State, Unit-1
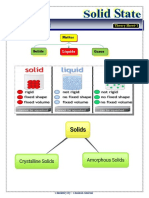
Solid, liquid and gas state of matter forms due to difference in force of attraction
between the particles. In solid state, there is strong force of attraction between
the particles. Due to strong attraction between the particles, solids are
rigid in nature and they have definite shape.
Stability of solid on the basis of thermal energy and intermolecular
forces: Due to vibratory motion, the particles of solids have thermal energy
and this thermal energy decreases the stability. Intermolecular forces increase
the stability of solids. If intermolecular force is more than thermal energy, then
solid will be more stable.
Q: Stability of a crystal is reflected in the magnitude of its melting
point'. Comment. (NCERT)
Answer: Solid with higher melting point have stronger force of attraction, so
they will be more stable.
Types of solids on the basis of arrangement of particles:
Crystalline solid: The solids in which the particles are arranged in a fixed
manner are called crystalline solids. These are called true solids. For example,
diamond and graphite are crystalline solids.
Amorphous solid: The solids in which the particles arranged randomly are
called amorphous solids. They are not true solids and are called supercooled
liquids. For example, plastic, rubber or glass are amorphous solids.
Uses of Amorphous Solids: 1. Glasses used in houses.
2. Rubber used in making shoes etc.
CRYSTALLINE SOLIDS AMORPHOUS SOLIDS
1. Particles are arranged in fixed Particles are not arranged in fixed
manner. manner.
2. Due to regular arrangement of Amorphous solids show short range of
particles over a long distance, order due to regular arrangement of
crystalline solids show long range of particles for a short distance.
order.
3. Particles have regular shape. Particles do not have regular shape.
4. High melting point. Low melting point.
5. They are anisotropic. They are isotropic.
6. They are true solids. They are pseudo or supercooled solids.
7. They show cleavage property due They do not show cleavage property
to sharp cutting edge. due to rough cutting edge.
Long range order: Crystalline solids have long range order because the
particles are arranged in regular manner for a long distance. In this
arrangement, we can judge the arrangement of particles if we know the position
of particles at other point.
Short range order: Amorphous solids have short range order because the
particles are arranged in regular manner for only a short distance.
We cannot judge the arrangement of particles even if we know the arrangement
of particles at other point.
1 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Difference between glass from a solid such as quartz: In glass, the
particles have short range order. In quartz, the constituent particles have both
long range and short range orders. Quartz can be converted into glass by
heating and then cooling it rapidly.
Crystalline solids have higher melting point than amorphous solids: Due
to regular arrangement of particles in crystalline solid, the force of attraction
between them is higher than the amorphous solids, so the crystalline solids have
higher melting point.
Isotropy: It is a property of amorphous solids. Amorphous solids have similar
value of electrical/thermal conductivity or refractive index in all the directions
due to same number of particles in every direction. The substances which
have the property of isotropy are called isotropic.
Anisotropy: It is a property of crystalline solids. Crystalline solids have
different values of electrical conductivity or refractive index in different direction
due to different number of particles in different directions. The
substances which have the property of anisotropy are called anisotropic.
Ques: Refractive index of a solid is observed to have the same value
along all directions. Comment on the nature of this solid. Would it show
cleavage property? (NCERT)
Answer: Amorphous solids show same value of refractory index or electrical
conductance in all directions due to same number of particles in each direction.
Amorphous solids do not show cleavage property because upon cutting with
knife, rough edges are observed.
Glass is amorphous solid and considered super cooled liquid: Glass has a
tendency to flow like liquids, so the glass is considered as a super cooled liquid.
The bottom of the old glass windows and doors are slightly thick at the
bottom and this proves that glass flows like liquid.
Supercooled liquids or pseudosolids: Amorphous solids are called
supercooled liquids because they are made up of thick liquid which changes into
solid at very low temperature. For example, the glass is a supercooled liquid. It
can be proved by the fact that the bottom of old window glasses are more thick
than the top of glass and it shows that glass has some fluid nature.
Crystallites: In the amorphous solids, there may be some part which looks like
crystalline solid and this part is called crystallites.
Glass of old building looks milky white: The glass is amorphous solid. Due
to the annealing (heating in day and cooling in night), the glass changes into the
crystalline solids and looks like milky white.
Types of crystalline solids?
1. Ionic solids. 2. Molecular solids. 3. Covalent solids. 4. Metallic solids.
1. Ionic Solids: The ionic solids are made up of positive and negative ions.
For example, NaCl is ionic solid which is made up of Na+ and Cl‒. There is
electrostatic force of attraction between the ions. Ionic solids have high
melting point due to strong force of attraction between the positive and negative
2 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
ions. In solid state, ions are not free, so ionic solids do not conduct electricity in
solid state. In molten state or in solution form, the free ions are present and
can conduct electricity. Due to the presence of positive and negative ions, they
are soluble in polar solvents.
2. Molecular Solids: The molecular solids are made up of molecules. They
are of three types.
a. Non-polar molecular solids: These solids are made up of non-polar
molecules such as Cl2, I2, H2 etc. These nonpolar molecules are bonded by
London force of attraction. It is a weak force and so these solids are soft.
They have no ions or free electrons, so they are not conductor of electricity.
b. Polar molecular solids: These solids are made up of polar molecules such
as HCl or SO2. The polar molecules have two poles, positive and negative.
There is dipole-dipole force of attraction between the molecules. These
are also nonconductor of electricity because there are no free ions or electrons.
3. Hydrogen bonded molecular solids: In these solids, there is hydrogen
bond between the molecules. The force of attraction between them is stronger
than London force of attraction. For example, molecules of water have H-bond.
4. Covalent or Network Solids: These solids are made up of nonmetal
atoms which are bonded to each other by covalent bonds. The covalent bond is
very strong bond and so the covalent solids are hard and have high melting
point. They do not conduct electricity. Diamond and graphite is example of
covalent or network solids. Graphite is conductor due to the presence of free
delocalized electrons.
5. Metallic Solids: A metallic solid is are made up of large number of
metallic ions which are held together by the sea of electrons. These
electrons are free and mobile. All the properties of a metal, like conductance of
electricity and heat, malleability, ductility and lustre is due to sea of electrons.
The force of attraction between the metallic ions is called metallic bond.
Metallic solids are very hard due to strong force of attraction between the
metallic ions.
Q: Solid A is a very hard electrical insulator in solid as well as in molten
state and melts at extremely high temperature. What type of solid is it?
Ans: It is a covalent or network solid. For example, diamond (C) and quartz
(SiO2).
Q: What type of solids are electrical conductors, malleable and ductile?
(NCERT)
Answer: Metallic solids are electrical conductors, malleable, and ductile.
Malleability and ductility of metals: Metallic solids are malleable and ductile
because by beating we can change the position of metallic ions without changing
the shape of the metallic crystal.
Electrical and thermal conductivity of metals: Free electrons present in
metallic solid absorb the heat and transfer this heat to other electrons, so
metallic solids are good conductor of heat and electricity.
3 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Kernels: Positively charged metallic ions are called kernels; these are formed
after donation of electron by metal atom.
Similarity between metallic and ionic solids: Both are solid due to strong
force of attraction and have high melting points.
Difference between metallic and ionic solids: Metallic crystals conduct
electricity by free electrons. Ionic crystal does not conduct electricity. Ionic
solids cannot be converted into sheet. Metallic crystals can be changed into
sheets and wires.
Hard and brittle nature of ionic solids: Ionic solids are hard due to strong
force of attraction between ions and they are brittle due to non-directional
bonds and break when force is applied.
Crystal lattice or space lattice: The three dimensional arrangement of
particles in crystalline solid is called crystal lattice.
Bravais lattice: All the 14 possible three dimensional lattices are called as
Bravais lattices.
Significance of a ‘lattice point’: Each lattice point represents one particle of a
solid which may be an atom, a molecule (group of atom), or an ion.
Unit Cell: The smallest but complete three dimensional unit of crystalline solid
is called unit cell. A large number of same unit cells combine to form the crystal
or space lattice.
They are of 2 types:
1. Primitive or Simple Unit Cells: The unit cells in which the particles are
present at the corners are called primitive unit cells.
4 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
2. Non-Primitive or Centered Unit Cells: The unit cells in which the particles
are present at the corners and at some other position also are called non-
primitive unit cells. These are of 3 types.
a. Face-Centered Unit Cells: In these unit cells, the particles are present at
the corner as well as at center each face of unit cells.
b. End-Centered or side centered Unit Cells: In these unit cells, the
particles are present at the corners as well as at the center of two opposite
faces.
c. Body-Centered Unit Cells: In these unit cells, the particles are present at
the corners as well as at the center of body.
d. Edge-Centered Unit Cells: In these unit cells, the particles are present at
the edges of unit cell. There are 12 edges in each unit cell.
Parameters of unit cell: (i). Edge length. (ii). Angles between the edges.
Formula of calculating contribution of each atom at different lattice
positions:
In a crystal lattice, any particle at the corner is shared by 8 unit cells, so the
contribution of each particle at corner is 1/8.
In a crystal lattice, any particle on the face is shared by 2 unit cells, so the
contribution of each particle at corner 1/2.
In a crystal lattice, any particle on the edge is shared by 4 unit cells, so the
contribution of each particle at corner 1/4.
In a crystal lattice, any particle within the body is shared by 1 unit cells, so the
contribution of each particle at corner 1.
Contribution of particles in simple unit cell: Total of 8 particles are present
in a unit cell on the 8 corners. Every particle at each corner is shared by 8 unit
cells, so contribution of each particle for unit cell is 1/8. Total contribution of all
the particles of corners for unit cell is 1.
Contribution of particles in BCC: Total number of atoms in one unit cell = 8
corners per corner atom + 1 body-centre atom
Contribution of particles in FCC: Total number of atoms in one unit cell = 8
corner atoms atom per unit cell + 6 face-centred atoms atom per unit cell
5 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Ques: Explain how much portion of an atom located at (i) corner
and (ii) body-centre of a cubic unit cell is part of its neighbouring unit
cell. (NCERT)
Answer: (i). An atom located at the corner of a cubic unit cell is shared by
eight unit cells. So, 1/8th portion of the atom is shared by one unit cell.
(ii). An atom located at the body centre of a cubic unit cell is not shared by its
neighbouring unit cell. So, its contribution to the unit cell is 1.
Ques: How many lattice points are in one unit cell of? (NCERT)
(i) Face-centred cubic. (ii) Face-centred tetragonal. (iii) Body-centred
Answer: (i) There are 14 (8 from the corners + 6 from the faces) lattice points
in face-centred cubic.
(ii) There are 14 (8 from the corners + 6 from the faces) lattice points in face-
centred tetragonal.
(iii) There are 9 (1 from the centre + 8 from the corners) lattice points in body-
centred cubic.
Coordination Number: It is the number of particles that touch a particle in
crystal lattice. For example, in NaCl each Na+ cation touches six Cl– anions and
each Cl– anions touches six Na+ cations, so coordination number in NaCl is six.
Closed packing: When the maximum available space is occupied by the
particles with minimum empty space, then it is called closed packing. There are
three types of closed packing.
1. Closed packing in one dimension: In this closed packing, the particles
are arranged in a row. Each particle touches two particles, so the coordination
number is 2.
Coordination number = 2
2. Closed packing in two dimensions: In this closed packing, the rows of
particles are placed over each other to form a sheet like structure. This type of
packing can be done in two types.
A. Square Closing Packing in Two Dimensions: In this close packing, the
particles of second row are placed on the particles of first row and making a
square structure. It is also called as AAA type arrangement because each layer
is similar to other layer. Coordination number in this arrangement is 4.
B. Hexagonal Close Packing in Two Dimensions (hcp): In this close
packing, the particles of second row are placed in the depression of first row and
making a hexagonal structure. It is also called as ABAB type arrangement.
In this type of packing, the coordination number is 6.
6 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
3. Closed packing in three dimensions: In this closed packing, the two
dimensional layers are arranged over each other. This packing can be done in
following types.
A. Square Closing Packing in three Dimensions: In this close packing, the
two dimensional layers are placed exactly on each other making a square
structure. It has AAA type arrangement. In this packing, the coordination
number is 6. This type of close packing will result in the formation of simple or
primitive unit cells.
Coordination number = 6
B. Hexagonal Close Packing (hcp) in three Dimensions by placing
particles of third layer over the tetrahedral voids: It is ABAB type
arrangement. In this close packing, the particles of first layer are placed in the
depression of second layer and the particles of third layer are placed in the
depression of second layer. Third row is exactly like the first row. In this only
26% space is empty and 74% space is covered.
In two dimensional hcp, the coordination number is 6 but in three dimensional
hcp, coordination number is 12. A single unit cell has 4 atoms in three
dimensional hcp.
C. Cubic Close Packing (ccp or fcc) in three Dimension by placing
particles of third layer over the octahedral voids: This packing forms ABC
ABC ABC pattern. In this type of packing, the particles of third layer are placed
over the octahedral voids of first layer that are not covered by second layer. This
packing gives face centered cubic, FCC, lattice. The packing efficiency is 74%
and free space available in this packing is 26% and coordination number is 12.
In two dimensional hcp, the coordination number is 6 but in three dimensional
hcp, coordination number is 12. A single unit cell has 4 atoms in three
dimensional hcp.
Body centred close packing (bcc): In this packing, one particle is present in
the centre of the unit cell and 8 particles are the corners. The coordination
number of central sphere is 8. The efficiency of this type of packing is less than
the hcp, fcc or ccp, only 68% space is occupied and 32% remains free.
Coordination number in three dimension packing:
(i) Simple cubic structure = 6. (ii) Face-centred cubic (FCC) = 12
(iii) Body-centred cubic (BCC) = 8
Interstices or voids: The holes or empty space present between the particles
in the crystal lattice are called interstices or voids. Types of voids: There are
two types of voids,
A. Tetrahedral voids: A void which is surrounded by 4 particles or spheres is
called tetrahedral void.
7 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
It is formed when the spheres of second layer are placed over the void of first
layer, then tetrahedral voids is formed. There are two tetrahedral voids per
atom of crystal.
Number of tetrahedral voids = 2 × Number of particles in close packing.
B. Octahedral Voids: A void which is surrounded by 6 particles or spheres is
called octahedral void. There is 1 octahedral void per atom.
Number of octahedral voids = Number of close-packed particles
Octahedral void forms when the particles of third layer are placed over
the void of second layer.
Question: A compound forms hexagonal close packed structure. What is
the total number of voids in 0.5 mol of it? How many of these are
tetrahedral.
Ans: We know that number of octahedral voids is equal to the number of
particles in close packing and tetrahedral voids are double the number of
particles. So, 0.5 mol of compound will have 0.5 mol or 3.011 x 1023 octahedral
holes and one mole or 6.023 x 1023 tetrahedral holes. A total of 1.5 moles of
holes will be there in 0.5 mol of compound.
Question: A compound is formed by two elements X and Y. The atoms of
element X form hcp lattice and those of element Y occupy ¼th of the
tetrahedral voids. What is the formula of the compound formed?
Answer: It is known that the number of tetrahedral voids formed is equal to
twice the number of atoms of element X.
It is given that only 1/4th of the tetrahedral voids are occupied by the atoms of
element Y.
Therefore, ratio of the number of atoms of X and Y = = 2: 1
So, the formula of the compound formed is X2Y
Each unit cell of CCP or FCC structure has 4 atoms, so it will have 4
octahedral voids and 8 tetrahedral voids.
Q: A cubic solid is made of two elements P and Q. Atoms of Q are at the
corners of the cube and P at the body-centre. What is the formula of the
compound? What are the coordination numbers of P and Q? (NCERT)
Answer: It is given that the atoms of Q are present at the corners of the cube.
So, number of atoms of Q in one unit cell= 8 x 1 = 1
8
It is also given that the atoms of P are present at the body-centre. Therefore,
number of atoms of P in one unit cell = 1. This means that the ratio of the
8 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
number of P atoms to the number of Q atoms, P:Q = 1:1. Hence, the formula of
the compound is PQ. The coordination number osf both P and Q is 8.
Question: If three elements A, B and C crystallize in a cubic solid lattice
with A atoms at the corners, B atoms at the body centre and C atoms at
the centre of the faces of the cube, then write the formula of the
compound.
Answer:
Q: A compound is formed by two elements M and N. The element N
forms ccp and atoms of M occupy 1/3rd of tetrahedral voids. What is the
formula of the compound?
Answer: The ccp lattice is formed by the atoms of the element N. The number
of tetrahedral voids formed is equal to twice the number of atoms of the element
N.
According to the question, the atoms of element M occupy 1/3rd of the
tetrahedral voids. So, the number of atoms of M is equal to of the
number of atoms of N.
So, ratio of the number of atoms of M to that of N is M: N . The
formula of the compound is M2 N3.
Question: A compound is formed by two elements A and B. Atoms of
the element B (as anions) make ccp and those of the element A (as
cations) occupy all the tetrahedral voids. What is the formula of the
compound?
Ans: As ccp lattice is formed by the element B, the number of tetrahedral voids
generated= 2 × number of atoms of B present in it.
Since, all the tetrahedral voids are occupied by the atoms of A.
2A =B]
So, the atoms of elements B and A are present in the ratio = 1: 2
So, the formula of compound is A2B.
Q: Ferric oxide crystallises in a hexagonal close-packed array of oxide
ions with two out of every three octahedral holes occupied by ferric
ions. Derive formula of ferric oxide. (NCERT)
Answer: Let the number of oxide (O2−) ions be x.
So, number of octahedral voids = x
9 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
It is given that two out of every three octahedral holes are occupied by ferric
ions.
So, number of ferric (Fe3+) ions
So, ratio of the number of Fe3+ ions to the number of O2− ions,
Fe3+: O2− = 2:3
Hence, the formula of the ferric oxide is Fe2O3.
Ques: A cubic solid is made of two elements X and Y. Atoms Y are at the
corners of the cube and X at the body centre. What is the formula of the
compound?
Sol: Contribution of atom at the centre of unit is one.
The contribution of atoms at the corners is also one.
So, the formula of the given compound is XY.
Packing efficiency or packing fraction: The total space occupied by the
particles of a crystal in cube is called packing efficiency or packing fraction.
Packing efficiency in simple cubic structures: Let ‘a’ be the cube edge and
‘r’ the atomic radius.
Since one atom is present in a unit cell, its volume
Therefore, 52% of unit cell is occupied by atoms and the rest 48% is empty
space.
(b) Packing efficiency in FCC, CCP or HCP structures: The efficiency of ccp
and hcp is 74%. We will calculate the packing efficiency of ccp. Unit cell length
is ‘a’. In this figure other sides are not shown to make it simple.
10 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Therefore, 74% of unit cell is occupied by atoms and the rest 26% to empty
space.
Question: Gold (atomic radius = 0.144 nm) crystallises in a face-centred
unit cell. What is the length of a side of the cell? (NCERT)
Answer: For a face-centred unit cell,
It is given that the atomic radius, r = 0.144 nm, so = 0.407
nm
So, length of a side of the cell = 0.407 nm
Question: Aluminium crystallises in a cubic close-packed structure. Its
metallic radius is 125 pm. (NCERT)
(i) What is the length of the side of the unit cell?
11 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
(ii) How many unit cells are there in 1.00 cm3 of aluminium?
Answer: (i) For cubic close-packed structure:
= 353.55 pm= 354 pm (approximately)
(ii) Volume of one unit cell = (354 pm)3= 4.4 × 107 pm3= 4.4 × 107 ×
10−30 cm3= 4.4 × 10−23 cm3
Therefore, number of unit cells in 1.00 cm3 = = 2.27 × 1022
(c) Packing efficiency in bcc structures: In this case, the sphere (or atom)
at the centre is in touch with other two atoms which are diagonally arranged.
,
The length of the body diagonal c is equal to r being the radius of the sphere. As
all the three spheres along the diagonal touch each other.
As already discussed, the total number of atoms associated in a bcc unit cell is
2, therefore, the volume (v) is,
12 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
68% of unit cell is occupied by atoms and the rest 32% us empty space.
Question: Niobium crystallises in body-centred cubic structure. If
density is 8.55 g cm−3, calculate atomic radius of niobium using its
atomic mass 93 u.
Answer: Density of niobium, d = 8.55 g cm−3
Atomic mass, M = 93 gmol−1.
As the lattice is bcc type, the number of atoms per unit cell, z = 2.
a3 = 3.612 × 10−23 cm3
a = 3.306 × 10−8 cm
For body-centred cubic unit cell:
= 1.432 × 10−8 cm= 14.32 × 10−9 cm= 14.32 nm
Question: An element of body-centred cubic (bcc) structure has a cell
edge of 288 pm. The density of the element is How many
atoms are present in 208 g of the element?
Ans: Volume of 208 g of the element
13 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Question: The density is . The length of edge of unit cell is 654
pm. Predict the type of cubic lattice to which unit cell of KBr
belongs.
Answer:
Because Z=4, so it is FCC.
Ques: An element with molar mass 2.7 × 10-2 kg mol-1 forms a cubic
unit cell with edge length 405 pm. If its density is 2.7 × 103 kg m−3,
what is the nature of the cubic unit cell? (NCERT)
Answer: It is given that density of the element, d = 2.7 × 103 kg m−3. Molar
mass, M = 2.7 × 10−2 kg mol−1 and edge length, a = 405 pm = 405 × 10−12 m =
4.05 × 10−10 m. It is known that, Avogadro’s number, NA = 6.022 × 1023 mol−1
Applying the relation,
This shows that four atoms of the element are present per unit cell. So, the unit
cell is face-centred cubic (fcc) or cubic close-packed (ccp).
Ques: An element with density 11.2g cm–3 forms f.c.c. lattice with edge
length of 4 × 10–8cm. Calculate the atomic mass of the element.
Ans: For fcc lattice number of atoms per unit cell, z = 4
a = 4 × 10–8cm, d = 11.2g cm–3, NA= 6.022 × 1023mol–1
M = d x a3 x NA
Z
M = 11.2 x (4 × 10–8cm)3 x 6.022 × 1023 = 107.9 g mol–1 or 107.9 u
4
14 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Question: Silver crystallises in fcc lattice. If edge length of the cell is
4.07 × 10−8 cm and density is 10.5 g cm−3, calculate the atomic mass of
silver. (NCERT)
Answer: a = 4.077 × 10−8 cm. d = 10.5 g cm−3 Z = 4
NA = 6.022 × 1023 mol−1
Using the relation:
= 107.13 g mol−1
So, atomic mass of silver = 107.13 u
Question: Copper crystallises into FCC lattice with edge length 3.61 ×
10−8 cm. Show that the calculated density is in agreement with its
measured value of 8.92 g cm−3.
Answer: Edge length, a = 3.61 × 10−8 cm.
As the lattice is fcc type, the number of atoms per unit cell, z = 4. Atomic
mass, M = 63.5 g mol−1
We also know that, NA = 6.022 × 1023 mol−1
Applying the relation:
= 8.97 g cm−3
The measured value of density is given as 8.92 g cm−3. So, the calculated
density 8.97 g cm−3 is in agreement with its measured value.
Ques. Iron has a body-centred cubic unit cell with a cell edge of 286.65
pm. The density of iron is 7.87g cm–3. Use this information to calculate
Avogadro’s number (At. mass of Fe = 56g mol–1).
15 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Ans:
Q21. The density of copper metal is 8.95 g/cm3. If the radius of copper
atom is 127.8 pm, is the copper unit cell a simple cubic, a body-centred
cubic or a face-centred cubic structure? (Given: At. mass of Cu=63.54
g/mol ).
Ans:
Ques. The well-known mineral fluorite is calcium fluoride. It is known
that in the unit cell of this mineral there are 4 Ca2+ions and 8F–ions and
the Ca2+ions are arranged in a face-centred cubic lattice. The F–ions fill
all the tetrahedral holes in the fcc lattice of Ca2+ions. The edge of the
16 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
unit cell is 5.46 × 10–8cm in length. The density of the mineral is 3.18g
cm–3. Use this information to calculate Avogadro's number. (Molar mass
of CaF2 = 78.08 g mol–1)
Ans: d = 3.18 g cm–3, Z = 4, a = 5.46 × 10–8cm
M = 78.08 g mol–1
We know that, d = ZxM So, Na = Z x M
Na x a3 a3 x d
Na = 4 x 78.08
(5.46 x 10-8)-3 x 3.18
Na = 313.32 x 1024
517.61
Na= 6.035 × 1023 mol-1
Ideal crystal: Any crystal in which all the particles at proper position is called
ideal crystal. It is found at absolute zero temperature which is 0 K or ‒273
degree Celsius. Above the absolute zero temperature, the particles of
crystal change the position due to increase in thermal energy.
Crystal Defect: Change or deviation from the normal arrangement of particles
in a crystal lattice is called crystal defect. The defects in the crystal are divided
into two types.
1. Point Defect: When any particle is missing or present in the interstitial
space of the crystal, then it is called point defect.
2. Line Defect: When the particles are missing or present in the interstitial
space in a line or row of the crystal, then it is called line defect.
There are 3 types of point defects.
1. Stoichiometric Defect. 2. Non-stoichiometric Defect. 3. Impurity
Defect.
A. Stoichiometric Defect (Also called intrinsic or thermodynamic
defects): In this defect, cation to anion ratio remains the same. Because there
is no change in the anion to cation ratio, the matter with this defect remains
natural.
17 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Vacancy Defect: When any particle is missing in the crystal, then it is called
vacancy or vacant defect. Due to missing of particles, the density of crystal
decreases in this type of defect.
Interstitial Defect: When some particle is present in the interstitial space of
the crystal, then it is called interstitial defect. Due to presence of extra particle
in the crystal lattice, the density of the crystal increases in this type of defect.
Schottky Defect: This type of defect is present in the ionic crystals. In
this defect, the equal number of anions and cations are missing. This defect is
present in the ionic solids in which the size of cation and anion is almost similar.
Crystal remains electrically natural. The density of the crystal decreases in this
defect due to missing of ions from crystal lattice. NaCl, KCl, CsCl, etc.
Frankel or Dislocation Defect: In this defect, the ion misses from its normal
position and goes in the interstitial space. As the number of ions remains same
within the crystal, the density or conductivity does not change. For example,
AgCl, AgBr, AgI, and ZnS show this type of defect.
Ionic solids containing large differences in the sizes of ions show this type of
defect. The smaller ion moves into the interstitial site.
B. Non-Stoichiometric Defect: In this defect, there is difference in the
anions to cations ratio. Anion or cation is missing from the crystal site. The
substances which have non-stoichiometric defect are called berthollides.
These are of 2 types, metal excess and metal deficient defect.
18 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Metal Excess Defect: In this type of defect, the positive ions are present in
excess.
There are 2 types of metal excess defects.
1. Anion Vacancy Defect: In this defect, the anions are missing from their site
and the electrons of anions occupy those sites. The solid remains neutral and
does not conduct electricity because the negative charge electrons fill the space
of negatively charged anions.
For example, when NaCl is heated in the presence of sodium vapour, the sodium
atoms collect on the surface of NaCl. The Cl- anion of NaCl becomes free and
combines with vapour sodium atom to form NaCl and give one electron. The
free electron produced in this process fits in the anion vacancy to form F-Centre.
This F-center is responsible for the yellow color of NaCl, violet colour in
KCl and pink colour of LiCl.
F-Center: When anion is absent from the lattice site and the electron fills that
site, then this site having electron is called F-center. This F-center is responsible
for the color in the crystals by absorbing energy of light. The yellow color of the
NaCl is due to the presence of F-center in the NaCl crystal.
2. Excess cation defect: In this defect, the extra cations are present in the
interstitial sites. But the solids with this type of defect remain neutral due to the
presence of electron in another interstitial site.
For example, on heating ZnO loses oxygen. Zinc changes into zinc ion by giving
electron. These free electrons move in the interstitial site. These free electrons
absorb the heat and move to higher energy level. When they return to normal
level, yellow colour appears. This is also known as metal excess defect.
In this defect, free electrons are present in the interstitial site and crystal with
this defect can conduct electricity. But due to presence of less number of
free electrons in this defect, solids with this type of defect are not good
conductor and are called semiconductor. These conductors are also
called n-type semiconductor because the flow of current is in normal
way by free electrons.
19 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Metal Deficiency Defect: In this defect, less number of positive ions are
present than negative ions. These are of 2 types.
1. Cation Vacancy Defect: In this defect, the cation is absent from the lattice
sites. When cation is missing from crystal, negative charge increases and to
balance the negative charge, some cations acquire more positive charge by
losing electron. This defect is shown by those metals (transition metals) which
have variable oxidation states.
For example, ferrous sulphide, FeS, has such type of defect. The Fe or iron has
two types of oxidation states like Fe2+ and Fe3+.
2. Extra anion Defect: In this defect, extra anion is present in the interstitial
sites. Extra negative charge is balanced by extra positive charge on some other
metal ions. Due to the metal deficiency defect P-type conductor forms
due to the formation of holes when metal ion acquires more positive
charge.
Impurity Defect: This type of defect occurs due to the presence of foreign
atoms in the crystal. The foreign atoms may replace the atoms of crystal or
may be present in the interstitial sites of crystal.
For example, if molten NaCl containing a little amount of SrCl2 is crystallised,
some of the sites of Na+ ions are occupied by Sr2+ ions. Each Sr2+ ion replaces
two Na+ ions, occupying the site of one ion, leaving the other site vacant. The
cationic vacancies thus produced are equal in number to those of Sr2+ ions.
When the foreign atoms replace the atoms of crystal, then the crystal formed is
called substitution solid. When the foreign atoms are present in the interstitial
sites, then the crystal formed is called interstitial solid.
Doping: Process of adding impurities to the crystalline substance is called
Doping. This process is done to change the property of crystals. For example,
we can change any neutral substance into semiconductor by doping.
Q: Examine the given defective crystal
A+ B– A+ B– A+
B– 0 B– A+ B–
A+ B– A+ 0 A+
B– A+ B– A+ B–
Answer the following questions:
(i) What type of stoichiometric defect is shown by the crystal?
(ii) How is the density of the crystal affected by this defect?
(iii) What type of ionic substances show such defect?
Ans:
(i) Schottky defect
(ii) Decreases
20 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
(iii) This type of defect is shown by ionic compounds which have high
coordination number and small difference in size of cations and anions e.g. NaCl,
KCl etc.
Q: ZnO turns yellow on heating. Why ?
Ans: On heating ZnO, loses oxygen. Zinc changes into zinc ion by giving
electron. These free electrons move to the interstitial site. These free electrons
absorb the heat and move to higher energy level. When they return to normal
level, yellow colour appears. This is also known as metal excess defect.
Metallic and Electrolytic Conductor: In metallic conductors, flow of
electricity is due to the electrons. In ionic conductors, the flow of electricity is
due to the ions.
N-Type Semiconductors: In this semiconductor, the flow of current is in
normal way by free electrons. It is found in metal excess defect crystals.
They are formed by doping of the crystal of a group 14 element such as Si or Ge
with a group 15 element such as P or As (arsenic). Group 14 elements like
silicon or germanium have four electrons in the outermost shell. When group 15
element such as P or As (arsenic) is added, four electrons of group 15 elements
will make covalent bonds with four electrons of group 14 elements. One
electron of each atom of group 15 elements will be free and it will help in
conductivity.
p−TYPE SEMICONDUCTOR: In this semiconductor, the electric current flows
through the electron holes. These holes are produced due to the doping of the
crystal of a group 14 element such as Si or Ge, with a group 13 element such as
B, Al or Ga. It is found in metal deficiency defect crystals. Elements of 13
group have three electrons in the outermost shell and they make three covalent
bonds with group 14 elements. A hole will form at the place of fourth electron in
group 13 element. This hole has positive charge and it will attract electron from
any other atom near it. In this process, the hole will become in other atom, so
electron will move through the holes and it will conduct electricity by holes.
21 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Question: Why mixing of group 14 element mixing with group 15
element changes in N-type conductor:
Answer: Group 14 element has four electrons in the outermost shell and 15
group has 5 electrons in the outermost shell. When the crystal of 14-group
semiconductor is doped with group-15 elements (P, As, Sb or Bi), only four
valence electrons of the doped atoms involve in formation of covalent bonds.
The fifth electron is free and this fire electron conduct electricity.
Question: Non-stoichiometric cuprous oxide, Cu2O can be prepared in
laboratory. In this oxide, copper to oxygen ratio is slightly less than 2:1.
Can you account for the fact that this substance is a p-type
semiconductor? (NCERT)
Answer: In the cuprous oxide (Cu2O), some of Cu+ ions are replaced by
Cu2+ ions. Every Cu2+ ion replaces two Cu+ ions and creating holes and it
conducts electricity with the help of positive holes. So, the substance is a p-type
semiconductor.
Question: Analysis shows that nickel oxide has the formula Ni0.98O1.00.
What fraction of nickel exist as Ni2+ and NI3+ ions?
Ans: Formula of the compound is Ni0.98 O1.00.
Suppose the number of Ni2+ is X, so Ni3+ will be 0.98-X.
Total charge on nickel = charge on oxygen.
2X + 3(0.98 – X)= 2
2X + 2.94 – 3X = 2
X= 0.94.
Percentage of Ni2+ = 0.94 x 100 = 96%.
0.98
Percentage of Ni2+ = 100 – 96 = 4%.
Question: Classify the following as p-type or an n-type semiconductor:
(i) Ge doped with In (ii) B doped with Si. (NCERT)
Answer: (i) Ge (a group 14 element) is doped with In (a group 13 element).
Therefore, a hole will be created and the semiconductor generated will be a p-
type semiconductor.
(ii) B (a group 13 element) is doped with Si (a group 14 element). Thus, a hole
will be created and the semiconductor generated will be a p-type semiconductor.
Q: Explain how vacancies are introduced in an ionic solid when a cation
of higher valence is added as an impurity in it. (NCERT)
Answer: When a cation of higher valence is added to an ionic solid, the cation
of higher valence replaces more than one cation of lower valence and some
vacancies create. For example, when Sr2+ is added to NaCl, each Sr2+ ion
replaces two Na+ ions. One Sr2+ ion occupies the site of one Na+ ion and the
other site remains vacant.
Q: Ionic solids, which have anionic vacancies due to metal excess
defect, develop colour. Explain with the help of a suitable example.
(NCERT)
22 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Answer: Electrons fill the anionic sites. These electrons absorb energy from the
light and get excited. When these electron return to the ground state, they
produce colour. For example, when crystals of NaCl are heated in an
atmosphere of sodium vapours, the sodium atoms get deposited on the surface
of the crystal and the chloride ions from the crystal move to the surface to form
NaCl with the deposited Na atoms. During this process, the Na atoms on the
surface lose electrons to form Na+ ions and the released electrons move into the
crystal and occupy the vacant anionic sites. These electrons get excited by
absorbing energy from the light and produce yellow colour.
Ques. How would you account for the following? (NCERT)
(i) Frenkel defects are not found in alkali metal halides.
(ii) Schottky defects lower the density of related solids.
(iii) Impurity doped silicon is a semiconductor.
Ans: (i) Due to the large size of alkali metals, they cannot fit in the interstitial
site and do not show Frankel defect.
(ii) Schottky defect is found in ionic solids. Density lowers in this defect due to
missing some of the ions.
(iii) Silicon has very low conductivity. To increase its conductivity, silicon is
doped with impurity. When doped with electron-rich impurities such as P or As,
n-type semi-conductor is obtained and when doped with electron-deficient
impurities, p-type semi-conductor is obtained.
Question: In terms of band theory, what is the difference. (i) Between
a conductor and an insulator. (ii) Between a conductor and a
semiconductor (NCERT)
Ans: (i) In the conductor, the valence band is partially-filled or it overlaps with a
higher energy vacant conduction band.
(ii) In the insulator, the valence band is fully-filled and there is a large gap
between the valence band and the conduction band.
(iii) The valence band of a semiconductor is filled and there is a small gap
between the valence band and the next higher conduction band. So, some
electrons can jump from the valence band to the conduction band and conduct
electricity.
23 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
Q: A group 14 element is to be converted into n-type semiconductor by
doping it with a suitable impurity. To which group should this impurity
belong? (NCERT)
Answer: An n-type semiconductor conducts because of the presence of extra
electrons. So, a group 14 element can be converted to n-type semiconductor by
doping it with a group 15 element.
Conductors: These generally include metals. Their conductivity is of the order
of
Insulators: These are solids which have very low conductivity values ranging
from . These are called insulators.
Question: Why each electron act as small or tiny magnet?
Ans: Each electron has two types of motions, one is orbital motion around the
nucleus and spin around its own axis. Due to these motions, magnetic moment
develops in each electron and it act as small magnet.
PARAMAGNETIC SUBSTANCE: These substances have unpaired electrons and
these are attracted by a magnetic field. For example O2, Cu2+, Fe3+ and Cr3+.
Paramagnetic substances changes into magnet when magnetic field is applied,
but lose magnetism when the magnetic field is removed.
DIAMAGNETIC SUBSTANCE: These substances have paired electrons and
these are repelled by a magnetic field. Example, H2O, NaCl, C6H6. Due to the
pairing of electrons, the magnetic moment of each electron is cancelled by
magnetic moment of other electron.
FERROMAGNETIC SUBSTANCE: The substances that are strongly attracted by
magnetic field are called ferromagnetic substances. After applying magnetic
field, these substances changes into magnet permanently. Example, iron,
cobalt, nickel, gadolinium and CrO2-.
In solid state, the metal ions of ferromagnetic substances make small groups
which are called domains and each domain acts as a tiny magnet. When
magnetic field is not applied, the domains are arranged randomly and their
magnetic moments are cancelled by each other. But, when the substance is
placed in a magnetic field, all the domains arrange in the direction of the
magnetic field and strong magnetic effect is produced. This arrangement of
domains becomes permanent even after the removal of the magnetic field, so
the ferromagnetic substance becomes a permanent magnet.
Question: What is Domain?
Ans: Small group of metallic ions which act as small magnet is called domain.
FERRIMAGNETIC SUBSTANCES: In these substances, the domains are
aligned in parallel and anti-parallel directions in unequal numbers. These
substances have large number of domains but the effect of most of domains are
24 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
Solid State, Unit-1
cancelled and only small number of domains produce magnetic moment. These
substances are attracted weakly by the magnetic field.
Examples include Fe3O4 (magnetite), ferrites such as MgFe2O4 and ZnFe2O4.
ANTI-FERROMAGNETIC SUBSTANCES: These substances have large number
of domains but the effect of each domain is cancelled by other domain. They
have zero magnetic moment because equal amount of domains are present in
opposite direction.
Curie-Temperature: The temperature at which ferromagenetic substance
becomes paramagnetic is called curie-temperature. For iron the curie-
temperature is 1033 K, for Ni it is 629 K and for it is 850 K. Below this
temperature paramagnetic behaves as ferromagnetics.
Question: What type of substances would make better permanent
magnets, ferromagnetic or ferrimagnetic. Justify your answer.
Answer: Ferromagnetic substances would make better permanent magnets
because in this all the domains are arranged in a direction of magnetic field. In
the ferrimagnetic substance, when magnetic field is applied, domains are
arranged in parallel and anti-parallel direction unequally, so they make weak
magnet.
Ques. Pure silicon is an insulator. How does it behave as a
semiconductor on heating?
Ans. Si atom has four valence electrons that make covalent bonds and electrons
are not present for conductivity. On heating, some outermost shell electrons
become loose and silicon becomes semiconductor.
Question: Why are solids containing F-centre are paramagnetic and
coloured?
Ans: This is due to presence of unpaired electrons in the vacant sites of anions
which absorb light from the visible range and give colour.
25 Chemistry Notes by Sandeep Sir , Mobile# 9813795294
You might also like
- Us To Make Solids of Desired PurposeDocument5 pagesUs To Make Solids of Desired PurposeSam JonesNo ratings yet
- Solid StateDocument11 pagesSolid StateGomathi VarshiniNo ratings yet
- LP - GC - Nature of SolidsDocument9 pagesLP - GC - Nature of SolidsAllyza SobosoboNo ratings yet
- LP - GP - Coulomb's LawDocument9 pagesLP - GP - Coulomb's LawAllyza SobosoboNo ratings yet
- Properties and Types of SolidsDocument2 pagesProperties and Types of SolidsSaatwat CoolNo ratings yet
- Types of SolidDocument37 pagesTypes of Solidmjlngpogi.walangibaNo ratings yet
- Properties of SolidsDocument3 pagesProperties of SolidsJashmin LarozaNo ratings yet
- UNIT - II InorganicDocument28 pagesUNIT - II Inorganicharirajans71No ratings yet
- CH-1 (Xii)Document11 pagesCH-1 (Xii)Vijay KumarNo ratings yet
- Amorphous and Crystalline SolidsDocument8 pagesAmorphous and Crystalline SolidsAli ImranNo ratings yet
- Structure and Properties of Solids Helps Us To Make Solids of Desired PurposeDocument37 pagesStructure and Properties of Solids Helps Us To Make Solids of Desired PurposeSam JonesNo ratings yet
- A premium institute for CBSE, NEET & JEEDocument25 pagesA premium institute for CBSE, NEET & JEEDigvijay SolankiNo ratings yet
- Solidstate Part 1Document35 pagesSolidstate Part 1Gopikrishna ThirunavukarasuNo ratings yet
- SolidsDocument2 pagesSolidsNimz StudioNo ratings yet
- Classification and Properties of Crystalline and Amorphous SolidsDocument3 pagesClassification and Properties of Crystalline and Amorphous Solidsmillinagi95No ratings yet
- Rizal General Chemistry 2 q3 Slm4Document12 pagesRizal General Chemistry 2 q3 Slm4Darlene OpeñaNo ratings yet
- Properties of Crystalline and Amorphous SolidsDocument5 pagesProperties of Crystalline and Amorphous SolidsShin Se KyungNo ratings yet
- ES Module 3 - Quarter 1 - Types of SolidsDocument13 pagesES Module 3 - Quarter 1 - Types of SolidsAnalynAsuncionAtaydeNo ratings yet
- General Chemistry 2 - Q3 - SLM4Document11 pagesGeneral Chemistry 2 - Q3 - SLM4Jonnel RoqueNo ratings yet
- Intermolecular Forces of Liquids and Solids Solids and Their PropertiesDocument39 pagesIntermolecular Forces of Liquids and Solids Solids and Their PropertiesSTANNo ratings yet
- CHAPTER-solid State Part 1Document2 pagesCHAPTER-solid State Part 1Arpandeep KaurNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (The Solid State) Topic: Solids and Their ClassificationDocument4 pagesCbse Test Paper-01 CLASS - XII CHEMISTRY (The Solid State) Topic: Solids and Their ClassificationAtashImranNo ratings yet
- Genchem2 q3m2b Solids-And-imf FinalDocument50 pagesGenchem2 q3m2b Solids-And-imf Finalroxtonandrada8No ratings yet
- Solid StateDocument17 pagesSolid Statenoorunnisa0184No ratings yet
- Solid StateDocument49 pagesSolid Statekishangopi123No ratings yet
- Solid State Day 1Document8 pagesSolid State Day 1Rakesh SenNo ratings yet
- Classification of SolidsDocument5 pagesClassification of SolidsJesse Jones SeraspeNo ratings yet
- Chpter 1 SolidsDocument30 pagesChpter 1 SolidsRahil HassanNo ratings yet
- Lecture 4. Properties of SolidsDocument27 pagesLecture 4. Properties of SolidsAcademe HelperNo ratings yet
- Types and Properties of SolidsDocument2 pagesTypes and Properties of SolidsFaith DicdicanNo ratings yet
- Intermolecular ForcesDocument9 pagesIntermolecular Forces11-STEM 1 Penaso, Hannah Nicole V.No ratings yet
- Solid State 6 JulyDocument18 pagesSolid State 6 JulyQwertyNo ratings yet
- Chapter 3Document9 pagesChapter 3JeromeNo ratings yet
- The 4 States of MatterDocument4 pagesThe 4 States of MatterCynthia UnayNo ratings yet
- Intermolecular Forces of Liquids and Solids Solids and Their Properties PDFDocument13 pagesIntermolecular Forces of Liquids and Solids Solids and Their Properties PDFpieNo ratings yet
- General Chemistry 2 Q3 Week 2Document2 pagesGeneral Chemistry 2 Q3 Week 2jcjimz259No ratings yet
- Chemistry: Class - 12 SubjectDocument7 pagesChemistry: Class - 12 SubjectHemant YadavNo ratings yet
- Unit 1: The Solid StateDocument30 pagesUnit 1: The Solid StateDhwani KapoorNo ratings yet
- Classifying MatterDocument53 pagesClassifying MatterTanya MaheshwariNo ratings yet
- Local Media8298476867722663082 PDFDocument34 pagesLocal Media8298476867722663082 PDFRaquel AvilaNo ratings yet
- General Chemistry 2 Lesson 3 Types of SolidsDocument11 pagesGeneral Chemistry 2 Lesson 3 Types of SolidsYeji SeoNo ratings yet
- Properties of Solids: Crystalline vs AmorphousDocument9 pagesProperties of Solids: Crystalline vs AmorphousLester Nyel MirandaNo ratings yet
- Solids and Their PropertiesDocument31 pagesSolids and Their PropertiesTrexi Mag-asoNo ratings yet
- Material Downloaded From - 1 / 16Document156 pagesMaterial Downloaded From - 1 / 16Pralabh AgarwalNo ratings yet
- 12 Chemistry Notes ch01 The Solid State PDFDocument16 pages12 Chemistry Notes ch01 The Solid State PDFgaurav sahuNo ratings yet
- 1.solid StateDocument33 pages1.solid StateBHAVITH SD VNS 06No ratings yet
- Aerospce Batch 3 PHY 4201 Lecture 1Document26 pagesAerospce Batch 3 PHY 4201 Lecture 1Rasel khanNo ratings yet
- Target Publications Pvt. Ltd. Chapter 01: Classification of SolidsDocument14 pagesTarget Publications Pvt. Ltd. Chapter 01: Classification of SolidsSayyed SohelNo ratings yet
- Solids 150702201407 Lva1 App6892Document9 pagesSolids 150702201407 Lva1 App6892Samina ZaibNo ratings yet
- Chem ReviewerDocument3 pagesChem Reviewernashnitsuga20No ratings yet
- Unit III CrystollographyDocument56 pagesUnit III Crystollographymwita mwitaNo ratings yet
- GEN CHEM 2 LESSON 3 Intermolecular Forces of Solids and Their Properties1.1Document27 pagesGEN CHEM 2 LESSON 3 Intermolecular Forces of Solids and Their Properties1.1Loraine Castro0% (1)
- Intermolecular Forces of Liquids and Solids Solids and Their PropertiesDocument59 pagesIntermolecular Forces of Liquids and Solids Solids and Their PropertiesAndre Jose ErminoNo ratings yet
- I. The Kinetic Molecular Theory of MatterDocument5 pagesI. The Kinetic Molecular Theory of MatterKwon NieNo ratings yet
- Children Encyclopedia Chemistry: The World of KnowledgeFrom EverandChildren Encyclopedia Chemistry: The World of KnowledgeRating: 5 out of 5 stars5/5 (3)
- 5921apni KakshaDocument109 pages5921apni KakshaVimal PrasadNo ratings yet
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- CH 10Document57 pagesCH 10Akef AfanehNo ratings yet
- The Solid State PDFDocument69 pagesThe Solid State PDFVNS TechNo ratings yet
- The Solid State ModifiedDocument70 pagesThe Solid State ModifiedR220603 THAMMINENI MALLIKARJUNANo ratings yet
- 11 Alcohols, Phenol and Ether 28Document28 pages11 Alcohols, Phenol and Ether 28Aryan POONIANo ratings yet
- 10 HALOALKANES AND HALOARENES 31 With Not All Ncert, But Pyq 34Document34 pages10 HALOALKANES AND HALOARENES 31 With Not All Ncert, But Pyq 34Aryan POONIANo ratings yet
- 12th Computer Applications Book Back 1 Mark Questions Prepared by Mr. MaskDocument21 pages12th Computer Applications Book Back 1 Mark Questions Prepared by Mr. MaskRokith SNo ratings yet
- Science Quiz Contest (School)Document4 pagesScience Quiz Contest (School)Aryan POONIANo ratings yet
- 3 Electrochemistry 33Document33 pages3 Electrochemistry 33Aryan POONIANo ratings yet
- HazCom QuizDocument8 pagesHazCom QuizMax McguireNo ratings yet
- Those Who Serve - Stephen Melillo (Score)Document22 pagesThose Who Serve - Stephen Melillo (Score)Fernando RamiresNo ratings yet
- Electric Tool Parts List: Air Compressor 2007 - 04 - 02 Model EC 119 (E3)Document3 pagesElectric Tool Parts List: Air Compressor 2007 - 04 - 02 Model EC 119 (E3)Ivan GallegosNo ratings yet
- Quick Manual v2.3: Advanced LTE Terminal With Flexible Inputs ConfigurationDocument16 pagesQuick Manual v2.3: Advanced LTE Terminal With Flexible Inputs ConfigurationanditowillyNo ratings yet
- The State of Madflya Pradesh: Chaptee SeconDocument38 pagesThe State of Madflya Pradesh: Chaptee Seconnavdeep tiwariNo ratings yet
- Despiece Motor Briggs 14hp 400707122102Document58 pagesDespiece Motor Briggs 14hp 400707122102leinadNo ratings yet
- HTTP Sleekfreak Ath CX 81 3wdev CD3WD METALWRK GTZ075CE B65 7 HTMDocument14 pagesHTTP Sleekfreak Ath CX 81 3wdev CD3WD METALWRK GTZ075CE B65 7 HTMPavan SripadaNo ratings yet
- Digimerge DPV34DPC Data SheetDocument2 pagesDigimerge DPV34DPC Data SheetJMAC SupplyNo ratings yet
- Purifying Water: Study of MethodsDocument21 pagesPurifying Water: Study of MethodsRohit Thirupasur100% (2)
- Production of Pure Platinum and Palladium From DoreDocument4 pagesProduction of Pure Platinum and Palladium From DoreLựuLiềuLìNo ratings yet
- JTP-JTPR enDocument9 pagesJTP-JTPR enLukman DwiNo ratings yet
- PH103 Section U Recap NotesDocument54 pagesPH103 Section U Recap NotesLuka MegurineNo ratings yet
- 986.33 Mesofilos Aerobios-PetrifilmDocument1 page986.33 Mesofilos Aerobios-PetrifilmBleidy NieblesNo ratings yet
- Family Nomenclature and Same-Name Divinities in Roman Religion and MythologyDocument17 pagesFamily Nomenclature and Same-Name Divinities in Roman Religion and MythologyhNo ratings yet
- XFGRRRDocument100 pagesXFGRRRAbchoNo ratings yet
- BS SMP 7-8-9 Bab B. Inggris Sec. Text (Reading) (SB - Descriptive Text)Document5 pagesBS SMP 7-8-9 Bab B. Inggris Sec. Text (Reading) (SB - Descriptive Text)TeresaAvilla Ayuning BudiCayestuNo ratings yet
- Telemecanique Integral 32 Motor StarterDocument52 pagesTelemecanique Integral 32 Motor StarterJaime IxtaNo ratings yet
- Lesson 2.2 Graphing Linear Functions by The Point Plotting MethodDocument4 pagesLesson 2.2 Graphing Linear Functions by The Point Plotting MethodAliah GombioNo ratings yet
- Sumalinog Teodoro Jr. LPDocument5 pagesSumalinog Teodoro Jr. LPDAITO CHRISTIAN DHARELNo ratings yet
- Troubleshooting GuideDocument88 pagesTroubleshooting GuideFrancisco Diaz56% (9)
- Major Project ReportDocument49 pagesMajor Project ReportMohini BhartiNo ratings yet
- U1L9 Student Guide (1) - 1Document4 pagesU1L9 Student Guide (1) - 1jonahNo ratings yet
- IBPhysics Particle Practice KeyDocument2 pagesIBPhysics Particle Practice KeyOnur YavuzcetinNo ratings yet
- Methods of preparation and properties of amines, alkyl cyanides, nitro compounds and aromatic nitro compoundsDocument22 pagesMethods of preparation and properties of amines, alkyl cyanides, nitro compounds and aromatic nitro compoundsVanshika JainNo ratings yet
- Spring 2009 Midterm Opkst Mth601Document10 pagesSpring 2009 Midterm Opkst Mth601Khurram NadeemNo ratings yet
- Adobe Scan 11-Oct-2022Document5 pagesAdobe Scan 11-Oct-2022onkarkumarsolankiNo ratings yet
- Mineral Resources: Earth ScienceDocument20 pagesMineral Resources: Earth ScienceRegina Mae Narciso NazarenoNo ratings yet
- Sir James ChadwickDocument6 pagesSir James ChadwickMichael GuevarraNo ratings yet
- Capitulo 9 Incropera 4 EdDocument16 pagesCapitulo 9 Incropera 4 EdDaxon RodriguezNo ratings yet
- Iberchem - French: Code Item Name Code Item Name 35000 7600Document2 pagesIberchem - French: Code Item Name Code Item Name 35000 7600Noor AshrafiNo ratings yet