Professional Documents
Culture Documents
Ca Synth
Uploaded by
api-465421809Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ca Synth
Uploaded by
api-465421809Copyright:
Available Formats
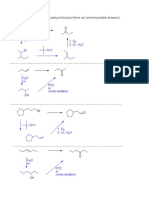
CARBOXYLIC ACID SYNTHESIS
a) Oxidation
i) 1° OH
O
Jones
R OH R OH
(Jones = CrO3, H2SO4, H2O)
* can use a variety of oxidants to get COOH (e.g. KMnO4, Tollen's, Fehling's, etc.)
remember: 2° OH goes to ketone not carbox. acid, and NR with 3°OH
ii) Aldehyde
O O
Jones
(or other strong ox. agent)
H R OH
b) Carbonation
O
1. Mg (or Li), Ether
RH2C X
2. CO2
3. H3O+ R OH
Stepwise: #1 makes grignard reagent (RMgX), #2 makes carboxylate ion, #3 protonates carboxylate
c) Nitrile Hydrolysis
O
1. NaCN
RH2C X
2. H+, H2O,
or R OH
2. NaOH, H2O,
3. H3O+
*without heat stops at amide
d) Oxidation of Aromatic Compounds O
1. KMnO4, H2O,
OH
2. H3O+
needs at least 1H at benzylic position
You might also like
- 118c Practice Synthesis KeyDocument18 pages118c Practice Synthesis Keyapi-465421809No ratings yet
- Phenol SynthDocument1 pagePhenol Synthapi-465421809No ratings yet
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDocument2 pagesOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- Ch 19 - Claisen Condensation and Diekmann CyclizationDocument68 pagesCh 19 - Claisen Condensation and Diekmann CyclizationBritany DyerNo ratings yet
- Zinc FinalDocument12 pagesZinc Finalamanuel tafeseNo ratings yet
- Ca SynthDocument1 pageCa Synthapi-465421809No ratings yet
- 118c Practice Synthesis KeyDocument18 pages118c Practice Synthesis Keyapi-465421809No ratings yet
- Phenol SynthDocument1 pagePhenol Synthapi-465421809No ratings yet
- 118c Practice Synthesis KeyDocument18 pages118c Practice Synthesis Keyapi-465421809No ratings yet
- Ca SynthDocument1 pageCa Synthapi-465421809No ratings yet
- Ca Rxns IIDocument1 pageCa Rxns IIapi-465421809No ratings yet
- Amide RxnsDocument1 pageAmide Rxnsapi-465421809No ratings yet
- Anhydride RxnsDocument1 pageAnhydride Rxnsapi-465421809No ratings yet
- Nitrile RxnsDocument1 pageNitrile Rxnsapi-465421809No ratings yet
- Phenol Rxns IDocument1 pagePhenol Rxns Iapi-465421809No ratings yet
- Ca RXN CheckDocument1 pageCa RXN Checkapi-4654218090% (1)
- Benzene RxnsDocument1 pageBenzene Rxnsapi-465421809No ratings yet
- Ester RxnsDocument1 pageEster Rxnsapi-465421809No ratings yet
- Ca Rxns IDocument1 pageCa Rxns Iapi-465421809No ratings yet
- Amine SynthDocument1 pageAmine Synthapi-465421809No ratings yet
- Acyl Halide RxnsDocument1 pageAcyl Halide Rxnsapi-465421809No ratings yet
- Haloalkanes and Haloarenes1Document15 pagesHaloalkanes and Haloarenes1Poorni RenuNo ratings yet
- AIEEE Chemistry Quick ReviewDocument1 pageAIEEE Chemistry Quick ReviewYashwanth KalyanNo ratings yet
- Chem-GA 1311 Spring 2021 F, ReductionDocument38 pagesChem-GA 1311 Spring 2021 F, ReductionHY-11 Đỗ Quốc TiệpNo ratings yet
- Organic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideDocument9 pagesOrganic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideAarya Nandal100% (1)
- Roadmap Problem - 9Document1 pageRoadmap Problem - 9abhyudaipathwayNo ratings yet
- Road Map (3) Problem: Aq. KOHDocument1 pageRoad Map (3) Problem: Aq. KOHShubham RajNo ratings yet
- Haloalkanes MADDocument31 pagesHaloalkanes MADggdfjkkvvNo ratings yet
- Hydrocarbon: Ashwani Tyagi Sir (Code: ATJEE)Document22 pagesHydrocarbon: Ashwani Tyagi Sir (Code: ATJEE)Prince DigvijayNo ratings yet
- Chapter 12 Lecture PDFDocument156 pagesChapter 12 Lecture PDFjoseph changNo ratings yet
- Introduction to Acids and BasesDocument102 pagesIntroduction to Acids and Basesjoseph changNo ratings yet
- 7 Coordination CompoundsDocument329 pages7 Coordination CompoundsArka100% (1)
- The Heck Reaction MechanismDocument11 pagesThe Heck Reaction MechanismPreeti YadavNo ratings yet
- ch15-16 SummaryDocument1 pagech15-16 Summaryapi-465421809No ratings yet
- Chapter 2 Lecture Slides PDFDocument108 pagesChapter 2 Lecture Slides PDFjoseph changNo ratings yet
- 1 Roh Carboxylic Acids: H CroDocument15 pages1 Roh Carboxylic Acids: H CroandrewwrobleNo ratings yet
- Alkene Addition ReactionsDocument179 pagesAlkene Addition Reactionsjoseph changNo ratings yet
- 6carboxylic AcidsDocument1 page6carboxylic AcidssharmimiameerasanadyNo ratings yet
- Chem 212 Alkyl Halide Problems 2Document1 pageChem 212 Alkyl Halide Problems 2kevinamy100% (1)
- Chem 212 Alkyl Halide Problems 4Document1 pageChem 212 Alkyl Halide Problems 4kevinamyNo ratings yet
- Haloalkanes and HaloarenesDocument26 pagesHaloalkanes and Haloarenesrajputrishi1982No ratings yet
- Roadmap Problem - 1Document1 pageRoadmap Problem - 1Siddharth SharmaNo ratings yet
- Alcohols Phenols EtherDocument55 pagesAlcohols Phenols EtherAnanya AgrawalNo ratings yet
- Aliphatic and Aromatic FlowchartDocument4 pagesAliphatic and Aromatic FlowchartapaperclipNo ratings yet
- Alcohols Ethers and Phenol-03 - Assignments (New)Document26 pagesAlcohols Ethers and Phenol-03 - Assignments (New)Raju SinghNo ratings yet
- Alkyl Halides and Aryl Halides SkillsDocument23 pagesAlkyl Halides and Aryl Halides SkillsNETHAKANI SUJATHA100% (1)
- CHAPTER25 HeterocyclesDocument44 pagesCHAPTER25 HeterocyclesRiyanto WidodoNo ratings yet
- Grignard Reagent Q.B.Document12 pagesGrignard Reagent Q.B.Aariya KumariNo ratings yet
- Practice TestDocument14 pagesPractice TestHimanshu JindalNo ratings yet
- General Organic Chemistry - Iii: Section (A) : Solvents, Reagents and Leaving GroupsDocument20 pagesGeneral Organic Chemistry - Iii: Section (A) : Solvents, Reagents and Leaving GroupsGOURISH AGRAWALNo ratings yet
- Reactions and Interconversions of Organic Functional GroupsDocument3 pagesReactions and Interconversions of Organic Functional Groupsmichelsonyip100% (1)
- Diazonium Salts, Azo DyesDocument8 pagesDiazonium Salts, Azo DyesDotsha Raheem100% (4)
- Diazonium Salts Azo DyesDocument8 pagesDiazonium Salts Azo DyesAnthony Basanta100% (1)
- Problem Set 10 KEYDocument3 pagesProblem Set 10 KEYCARLOS ALBERTO OSORIO MARTINEZNo ratings yet
- Chapter 9 Alcohol Reactions: I. Reactions of Alcohols With Acids and BasesDocument9 pagesChapter 9 Alcohol Reactions: I. Reactions of Alcohols With Acids and BasesRoberto SIlvaNo ratings yet
- Carbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)Document12 pagesCarbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)agrawaltwinkle2005No ratings yet
- Solutions Test 3Document4 pagesSolutions Test 3roorayNo ratings yet
- Chapter 9 Alcohol Reactions: I. Reactions of Alcohols With Acids and BasesDocument9 pagesChapter 9 Alcohol Reactions: I. Reactions of Alcohols With Acids and BasesRoberto SIlvaNo ratings yet
- Mechanism of Reaction of NBS and H2O2Document15 pagesMechanism of Reaction of NBS and H2O2N.GokilaNo ratings yet
- 29 Carboxylic Acids Formula Sheets QuizrrDocument8 pages29 Carboxylic Acids Formula Sheets QuizrrArjunNo ratings yet
- Orgo ReactionsDocument15 pagesOrgo ReactionsDaesikNo ratings yet
- STPM Chemistry Topic 17 Hydroxyl Compound (Short Notes)Document1 pageSTPM Chemistry Topic 17 Hydroxyl Compound (Short Notes)Chris Lau100% (1)
- Grignard Reagents Problem SetDocument9 pagesGrignard Reagents Problem SetDarsh ThiyagarajanNo ratings yet
- Chapter 8 Reactions of AlcoholsDocument12 pagesChapter 8 Reactions of AlcoholsRoberto SIlvaNo ratings yet
- Aromatic Chemistry Assignment #3 2018-2019 ANSWERSDocument5 pagesAromatic Chemistry Assignment #3 2018-2019 ANSWERSZoe NorvilleNo ratings yet
- Benzene RxnsDocument1 pageBenzene Rxnsapi-465421809No ratings yet
- Ester RxnsDocument1 pageEster Rxnsapi-465421809No ratings yet
- Amine SynthDocument1 pageAmine Synthapi-465421809No ratings yet
- Week 6 Practice Final Part 2 Chapter 18Document4 pagesWeek 6 Practice Final Part 2 Chapter 18api-465421809No ratings yet
- Acyl Halide RxnsDocument1 pageAcyl Halide Rxnsapi-465421809No ratings yet
- 118b mt1 Study Guide 2Document21 pages118b mt1 Study Guide 2api-465421809No ratings yet
- 118b mt2 Study Guide 2Document20 pages118b mt2 Study Guide 2api-465421809No ratings yet
- ch18 SummaryDocument1 pagech18 Summaryapi-465421809No ratings yet
- Week 5 Practice Final Part 1 - New Material 1Document3 pagesWeek 5 Practice Final Part 1 - New Material 1api-465421809No ratings yet
- Ca Rxns IDocument1 pageCa Rxns Iapi-465421809No ratings yet
- CH 17: Aldehydes and Ketones: Nasiri CHE118B Final Study Guide Spring 2019 - S. Ly Sdly@ucdavis - EduDocument17 pagesCH 17: Aldehydes and Ketones: Nasiri CHE118B Final Study Guide Spring 2019 - S. Ly Sdly@ucdavis - Eduapi-465421809No ratings yet
- Midterm 1 Practice QuestionsDocument8 pagesMidterm 1 Practice Questionsapi-465421809No ratings yet
- ch17 SummaryDocument1 pagech17 Summaryapi-465421809No ratings yet
- Resonance Practice ProblemsDocument12 pagesResonance Practice Problemsapi-465421809No ratings yet
- ch15-16 SummaryDocument1 pagech15-16 Summaryapi-465421809No ratings yet
- MOC Alcohol RXN Map PDFDocument2 pagesMOC Alcohol RXN Map PDFNickOoPandeyNo ratings yet
- 118 BprsynthDocument19 pages118 Bprsynthapi-465421809No ratings yet
- ch14 SummaryDocument1 pagech14 Summaryapi-465421809No ratings yet