Professional Documents
Culture Documents
Grignard Reagent Q.B.
Uploaded by
Aariya KumariOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Grignard Reagent Q.B.
Uploaded by
Aariya KumariCopyright:
Available Formats
ORGANIC CHEMISTRY
TARGET IIT JEE 2018
XII (ALL)
QUESTION BANK ON
GRIGNARD'S
REAGENT
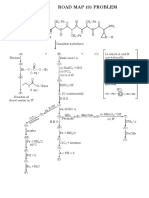
HOH or ROH
2
or Ph–OH
2 NH H
O2 ,H2 O
CdCited
N
SO2,H
lim
or R 3 or R
2 O,
CS H
l2
O lim gBr
NH
2 ,H
S,H
H2 ite 2
X
O, d
R–
H SiC
CO HC l
limit 4 CH 2
2 ,H O –CH=
RCHO,H2 ed
ClCH2
O R–Mg–X R–Mg–X
RCOR,H2O PbCl2 ClCH2 OR
Me–CH-CH2,
limited
H2O Ph
O HCO
H2 Cl 2 CH
O H 2, OEt Zn ted
3O
2C
HC (
-C i l
C l–
lim
eq.) 2 O
CH 2
OO e
H
RCO
CN
(½ Et,H
N,
2
Et q.)
Cl
½
RC
,H 2
I2
O Et
Br2
OO
O
RC
A diamond is merely a lump of coal that did well under pressure.
Q.1 The order of reactivity of alkyl halide in the reaction R–X + Mg RMgX is
(A) RI > RBr > RCl (B) RCl > RBr > RI (C) RBr > RCl > RI (D) RBr > RI> RCl
Q.2 Br–CH2–CC–CH2–Br Mg
BrMg–CH2–CC–CH2–MgBr
( excess)
Et 2O
Product
The major product is:
(A) Br–Mg–CH2–CC–CH2–Br (B) Cyclobutyne
(C) —(CH 2 C C CH 2—
)n (D) CH2 = C=C=CH2
Q.3 On conversion into Grignard followed by treatment with ethanol, how many alkyl halides (excluding
stereoisomers) would yield 2-methyl butane.
(A) 2 (B) 3 (C) 4 (D) 5
Q.4 Which of the following reacts with Grignard reagent to give alkane?
(A) nitro ethane (B) acetyl acetone (C) acetaldehyde (D) acetone
Q.5 How many litres of methane would be produced when 0.595 g of CH3MgBr is treated with excess of
C4H9NH2
(A) 0.8 litre (B) 0.08 litre (C) 0.112 litre (D) 1.12 litre
Q.6 How many litres of ethene would be produced when 2.62 g of vinyl magnesium bromide is treated with
224 ml of ethyne at STP.
(A) 0.224 litre (B) 0.08 litre (C) 0.448 litre (D) 1.12 litre
MgBr OH
Q.7 + A
O – Ph
(A) (B) (C) (D)
Q.8 In which of the following reactions 3°alcohol will be obtained as a product.
O
||
(A) MgBr (excess) + H C Cl
H
O
||
(B) PhMgBr (excess) + CH 3 C Cl
H
O O
|| ||
(C) CH3MgBr (excess) + CH 3 C O C CH 3
H
O
||
(D) CH3MgBr (excess) + Cl C O Et
H
QUESTION BANK ON GRIGNARD REAGENTS [2]
D O
Q.9 1
equivalent Mg
X 2
Y; Y is
ether
(A) (B) (C) (D) None of these
Q.10 Compounds are shown with the no. of RMgX required for complete reaction, select the incorrect option
(A) CH3COOC2 H5 1
(B) CH3COCl 2
(C) HOCH2COOC2H5 3
(D) 4
Q.11 What will be the order of reactivity of the following carbonyl compounds with Grignard's reagent?
H H CH3 Me3C
(I) C=O (II) C=O (III) C=O (IV) C=O
H CH3 CH3 Me3C
(A) I > II > III > IV (B) IV > III > II > I
(C) II > I > IV > III (D) III > II > I > IV
OH
Q.12 Carbonyl compound (X) + Grignard reagent (Y) Me Ph
Et
X , Y will be
O O
|| ||
(A) Et C Ph , Me Mg Br (B) Me C Ph , Et Mg Br
O O
|| ||
(C) Me C Et , Ph Mg Br (D) Et C Ph , Et Mg Br
Q.13 (R) - 2-Bromooctane (
i ) Mg
X
(ii ) CO 2
(iii ) H
X is
(A) (B)
(C) A and B both (D) None of these
QUESTION BANK ON GRIGNARD REAGENTS [3]
Q.14 In which one of the following reaction products are not correctly matched in
(A) RMgX + CO2
Carboxylic acid
(2) H
(B) RMgX + C2H5OH Alkane
(C) RMgX + CH3CH2Cl Alkene
(D) RMgX + Cl O Ether
Q.15 The number of moles of grignard reagent consumed per mole of the compound
is:
(A) 4 (B) 2 (C) 3 (D) 1
Q.16 Select the correct statement:
(A) 1,4-dibromobutane react with excess of magnesium in ether to generate di-Grignard reagent.
(B) 1,2-dichlorocyclohexane treated with excess of Mg in ether produces cyclohexene.
(C) Vicinal dihalides undergo dehalogenation to give alkene when heated with Zn dust or Mg.
(D) 1,3-dichloropropane by treatment with Zn dust or Mg forms cyclopropane.
O
||
CH3 C CH3
Mg H
Q.17 CH3–CH=CH2 Br
2
Dry ether NH 4Cl
End product of above reaction is
CH 2
||
(A) CH 2 CH CH 2 C CH 3 (B) H 2C CH CH C CH 3
|
CH 3
OH
|
(C) H 2C CH CH 2 C CH 3 (D) H 2C CH CH 2 CH CH 2 OH
| |
CH 3 CH 3
OEt
followed
Q.18 MgBr + H – C OEt product.
OEt H 3O
Ethyl ortho formate
O
||
(A) C CH 3 (B) CH2CH=O
(C) CH = O (D) CH CH 3
|
CH O
QUESTION BANK ON GRIGNARD REAGENTS [4]
Br
1. Mg / ether
Q.19 Product (s)
2. CH 3CHCH2 CH 3. H 3O
| ||
OH O
Select the product from the following
I: II : CH 3CHCH 2CH III :
| ||
OH O
(A) III (B) I, III (C) I, II (D) II, III
O
||
Q.20 C 2 H 5 O C OC 2 H 5 2
CH 3MgBr
A. Product A formed
(A) is ethyl acetate
(B) further react with CH 3MgBr/H2O+ to give acetone
(C) further react with CH3MgBr/H2O+ to give t-butyl alcohol
(D) Can give pinacol when treated with Mg followed by H2O
Q.21 Order of rate of reaction of following compound with phenyl magnesium bromide is:
O O O
|| || ||
Me C Cl Me C H Me C O Et
I II III
(A) I > II > III (B) II > III > I (C) III > I > II (D) II > I > III
Q.22 Select the correct order of decreasing reactivity of the following compounds towards the attack of
Grignard reagent
(I) Methyl benzoate (II) Benzaldehyde (III) Benzoylchloride (IV) Acetophenone
(A) II > III > I > IV (B) I > II > III > IV
(C) III > II > IV > I (D) II > IV > I > III
CH3MgX
Q.23 O NH4Cl Product is
(A) Enantiomer (B) Diastereisomer (C) Meso (D) Achiral
Q.24 Nucleophilic addition of Grignard reagent cannot occur in
O O O O
|| || || ||
(A) CH 3 C C CH 3 (B) CH 3 C CH 2 C CH 3
O O
|| || O
(C) CH 3 C CH 2 CH 2 C CH 3 (D)
O
QUESTION BANK ON GRIGNARD REAGENTS [5]
CH MgBr
Q.25 CH 3CCH 2CH 2CH 2Cl 3 A, A is
||
O
CH 3
|
(A) CH 3CCH 2CH 2CH 2Cl (B) CH 3CCH 2CH 2CH 2CH 3
| ||
OH O
(C) (D)
O O
|| ||
Q.26 CH 3CCH 2CH 2COCH 2CH 3 (
i ) CH 3MgBr ( one mol )
A, A formed in this reaction is
( ii ) H 3O
OH O O O
| || || ||
(A) CH 3CCH 2CH 2COCH 2CH 3 (B) CH 3CCH 2CH 2CCH 3
|
CH 3
CH 3 CH 3
| |
(C) (D) CH 3CCH 2CH 2CCH 3
| |
OH OH
Cl2 Mg 3 CH CHO
Q.27 PhCH3 (A) (B) (C)
h ether NH4 Cl
CH3
OH
| OH CH3 OH
CH2 – CH – CH3 CH3 |
CH – CH3 C – CH3
(A) (B) (C) (D)
CH CH3
HO CH3
QUESTION BANK ON GRIGNARD REAGENTS [6]
Q.28 Select the correct order of reactivity towards Grignard reagent for nucleophilic attack.
O O O
|| || ||
(A) R C O C R < R C H
(B) Cl CH 2 C H > CH 3CH 2 C H
|| ||
O O
O O
|| ||
(C) CH 3 C O NO2 < CH 3 C O
O O
|| ||
(D) R C OR > R C NH 2
Q.29 In the reaction sequence:
( i ) CH MgBr / CuCl
3 (X) Major + (Y)
( ii ) H 2O / H
(X) & (Y) respectively are
OH CH3 OH
(A) , (B) ,
CH3
(C) , (D) ,
(2)
O OH
C
Q.30 O (3)
RMgX
( 2 moles)
H
H
(1)
S
C
CH
(4)
Deprotonation will occur from the following positions:
(A) 1,2 (B) 1,3 (C) any two positions (D) 1,4
HO OEt
2 CH3MgX
Q.31 NH4Cl P 1 + P2
Identify P1 & P2.
O HO Me
(A) CH4 (B) (C) (D)
O
QUESTION BANK ON GRIGNARD REAGENTS [7]
Br
14
NaHCO
Q.32 Mg
(A) (
i ) CO 2
(B) 3 (C) gas
( ii ) H / H 2O
Product C is
(A) CO (B) 14CO
2
(C) CO2 (D) A mixture of 14CO and CO2
2
3 ( i ) CH ONH
Q.33 2CH3MgBr ( 2
ii ) H
(A) CH3–O–NH–CH3 (B) CH3–NH–CH3
(C) CH3–NH2 (D) CH3–OH
O OH
Q.34 (
i ) CH 3MgBr
(A)
( ii ) H / H 2 O
CH3
(A) The product is optically active
(B) The product contains plane of symmetry
(C) The product shows geometrical isomerism.
(D) The product shows optical isomerism.
Q.35 Which of the following is incorrect.
O
O
C ||
CH3MgX
(A) Cl OC2H5 (1 CH 3 C OC2 H 5
eq )
O
OC2H5 ||
C 2 H5MgX
(B) CH3–C OC2H5 CH 3 C OC2 H 5
(1eq )
OC2H5
S S
|| H3O ||
(C) CH3MgX + C = S CH 3 C SH
O O
|| ||
H3O
(D) CH3MgX + C = O CH3 C OH
Q.36 Which reaction gives 1° aromatic amine as major product.
(A) Mg
/
ether
NH
3 (B) Mg
/ ether
NH
3
(C) Mg
/
ether
NH
3 (D) Hg
(OAc ) 2
Ph NH 3 / NaBH 4
QUESTION BANK ON GRIGNARD REAGENTS [8]
O
||
Q.37 CH3MgBr + CH2=CH C H H Product (1, 4 addition). It is
3O
OH
|
(A) CH 2 CH C H (B) CH 2CH CH CH 3
| |
CH3 OH
(C) CH3CH2CH2CHO (D) none
Q.38 (
i ) PhMgBr
Product.
(ii ) NH 4Cl
Me
Products in this reaction will be
(A) Stereoisomers (B) Enantiomer (C) Diastereomers (D) Geometrical isomers
CH MgBr (1 eq .)
Q.39 3 ?
The product is:
(A) (B) (C) (D)
Q.40 CH2 = C = O (
i ) Br2
C4H8O
(ii ) CH 3MgBr
( 2 equi )
(A) (B) (C) (D) All of these
RMgX
Q.41 RMgX + CH3CN NH
4Cl
(A) (B) will be NH 4Cl
(A) 1° ROH (B) 2° ROH (C) 3° ROH (D) Alkene
QUESTION BANK ON GRIGNARD REAGENTS [9]
Q.42 CH3—CH—CH2 CH
3MgCl
H 2O
O
(A) CH 3 CH CH 2OH (B) CH 3 CH CH 2 CH 3
| |
CH 3 OH
(C) CH 3 CH CH 3 (D) HO – CH2 – CH2 – CH2 – CH2 – OH
|
CH 3
Q.43 The reaction of 1 mole each of p-hydroxy acetophenone and methyl magnesium iodide will give
O O
(A) CH4 + IMgO C—CH3 (B) CH3–O C—CH3
OMgI MgI
O
(C) CH3–C OH (D) CH3O C—CH3
CH3
r1
Q.44 (i) + Ph Mg Br
Ph CH2 CH2 OH
O
r2
(ii) + Ph Mg Br Ph CH2 CH2 CH2 OH
O
(A) r2 > r1 (B) r1 > r2 (C) r1 = r2 (D) r1 = 2r2
Q.45 How many moles of Grignard reagent will be required by one mole of given compound?
O
SH
HO C – OEt
C – Cl
CH2–CH2 O
Cl
(A) 7 (B) 6 (C) 8 (D) 5
Q.46 Consider the given organometallic compound.
(I) (CH3)2Hg (II) (CH3)2Zn (III) (CH3)2Mg (IV) CH3Li
The correct decreasing order of ionic character is
(A) I > II > III > IV (B) II > I > III > IV (C) I > III > II > IV (D) IV > III > II > I
QUESTION BANK ON GRIGNARD REAGENTS [10]
O (i) CH3MgBr
|| + P
(ii) H3O
Q.47 CH3CH = CH C CH 3
(i) CuI,CH3MgBr
+ Q
(ii) H3O
OH O
| ||
(A) P is CH3CH = CH C Me Q is CH 3CH CH 2 C CH3
| |
Me CH 3
O OH
|| |
(B) P is CH 3CH CH 2 C CH3 Q is CH3CH = CH C Me
| |
CH 3 Me
(C) P is CH 3CH CH C(CH 3 ) 2 Q is (CH 3 ) 2 CHCH 2C CH 3
| ||
OH O
(D) P is (CH 3 ) 2 CHCH 2C CH 3 Q is CH 3CH CH C(CH 3 ) 2
|| |
O OH
For Q. No.48 to Q. No. 50
Consider the given reaction and answer the following questions
COOCH3
MeMgBr
Products
O OCH3 (excess)
O SH
||
O
Q.48 No. of RMgX consumed in the reaction is
(A) 4 (B) 5 (C) 6 (D) 7
Q.49 How many product will be fromed in given reaction (excluding stereo)
(A) 2 (B) 3 (C) 4 (D) 5
Q.50 Which of the following reaction will give the same Hydrocarbon formed as one of the product in the
above reaction.
(A) EtMgBr + Me – OH (B) PhMgBr + Me – OH
(C) MeMgBr + Ph – OH (D) MeMgBr + CH3 – CHO
QUESTION BANK ON GRIGNARD REAGENTS [11]
ANSWER KEY
RH R–OH
H
RSO 2 RH R2Cd RSH
S
HOH or ROH
OH R2 Hg
2
or Ph–OH
R–C–SH
2 NH N
RCH 2
O2,H2 O
R–R
H
SO2,H
CdC
or R 3 or R
2 O,
O CS
l2
O Hg
OH
NH
2 ,H
S,H
H2 R 2CH R Si Br
X
O,
R–
R–C–OH 4 2
R–CH2 CH=CH2
H
CO HC SiCl
2 ,H O =CH 2
RCHO,H2 4
ClC H2–CH
R–CH2–CH–Me O R–Mg–X PbR4 R–Mg–X
Me–CH-CH2, RCOR,H2O PbCl 2 ClCH2OR
RCH2OR
HO H2O RC Cl
O HCO 3 O l CO
O CH 2,
H2 OEt H nC 2
3O
Z OE
HC (
- t
Cl–
eq.) 2 O
CH 2
OO ½ e
H
RCO
CN
(½ Et,H
N,
R–COOEt
2
Et q.)
Cl
R–CH2–CH2 –OH R–CHO R2Zn
RC
,H 2
I2
OEt H
Br2
OO
O
RC
R2–C=O OH RCl R–CN
OH R–CH2
O RBr R–I
R–C–R
R–C
R
R
Q.1 A Q.2 D Q.3 C Q.4 A,B Q.5 C
Q.6 C Q.7 A Q.8 B,C,D Q.9 D Q.10 A
Q.11 A Q.12 A,B,C Q.13 C Q.14 C Q.15 A
Q.16 A,C,D Q.17 B Q.18 C Q.19 C Q.20 C,D
Q.21 A Q.22 C Q.23 A Q.24 B,D Q.25 C
Q.26 C Q.27 A,B,C Q.28 B Q.29 B Q.30 A
Q.31 A,B Q.32 C Q.33 C,D Q.34 B,C Q.35 B
Q.36 B Q.37 C Q.38 A,C,D Q.39 D Q.40 A
Q.41 C Q.42 B Q.43 A Q.44 B Q.45 A
Q.46 D Q.47 C Q.48 C Q.49 C Q.50 C
QUESTION BANK ON GRIGNARD REAGENTS [12]
You might also like
- Chapter36 - BiomoleculesDocument19 pagesChapter36 - BiomoleculesAkash GoelNo ratings yet
- IIT-JEE Chemistry by N.J. sir: Aldehydes and KetonesDocument10 pagesIIT-JEE Chemistry by N.J. sir: Aldehydes and KetonesMahendra ChouhanNo ratings yet
- IIT-JEE ChEmistry by N.J. sir: ORGANIC ChemIstRyDocument32 pagesIIT-JEE ChEmistry by N.J. sir: ORGANIC ChemIstRysachin pantNo ratings yet
- Basara Vidyakshetram, Madhapur: Na/dry - Et ODocument8 pagesBasara Vidyakshetram, Madhapur: Na/dry - Et OvardeshNo ratings yet
- Maximizing Marks in Chemistry SectionsDocument12 pagesMaximizing Marks in Chemistry SectionsGovind SajuNo ratings yet
- BJ Chemistry Kinetics ExerciseDocument25 pagesBJ Chemistry Kinetics Exercisethevamayan100% (1)
- Alcohol SheetDocument25 pagesAlcohol SheethtethddthdhtNo ratings yet
- 9.GOC & IsomerismDocument37 pages9.GOC & IsomerismVinod AgrawalNo ratings yet
- Vidyamandir Classes: Organic Chemistry RevisionDocument66 pagesVidyamandir Classes: Organic Chemistry Revisionansh guptaNo ratings yet
- 13 05 13 Chemistry Electrochemistry Assignment 3Document7 pages13 05 13 Chemistry Electrochemistry Assignment 3Gadde Gopala KrishnaNo ratings yet
- Atp Star DPP Jee AdvDocument100 pagesAtp Star DPP Jee AdvrajNo ratings yet
- S-Block Bansal PDFDocument20 pagesS-Block Bansal PDFAshish RanjanNo ratings yet
- Carbonyl Compounds - XII PDFDocument39 pagesCarbonyl Compounds - XII PDFMathoholic OjaNo ratings yet
- Etoos 9 PDFDocument24 pagesEtoos 9 PDFB. P. A Music INDIA100% (1)
- ACA-3B Full Inorganic Chemistry Class (11+12) (152 Questions+Answers)Document16 pagesACA-3B Full Inorganic Chemistry Class (11+12) (152 Questions+Answers)Biswajit GhoshNo ratings yet
- Goc & Eas Test-IiDocument7 pagesGoc & Eas Test-IiAniket GuptaNo ratings yet
- Iupac and Goc - With KeyDocument48 pagesIupac and Goc - With KeyDj chemzoneNo ratings yet
- Reaction IntermediatesDocument32 pagesReaction Intermediatestechno studioNo ratings yet
- MKA SIR REACTION MECHANISM EXERCISE NOTESDocument39 pagesMKA SIR REACTION MECHANISM EXERCISE NOTESMrigank GuptaNo ratings yet
- Question Bank MOD and Indefinite IntegrationDocument15 pagesQuestion Bank MOD and Indefinite IntegrationRAKTIM ROUTHNo ratings yet
- Question PaperDocument15 pagesQuestion PaperSidNo ratings yet
- Alcohol Phenol & Ether Class-12 Jee Package PDFDocument111 pagesAlcohol Phenol & Ether Class-12 Jee Package PDFPrathmesh DixitNo ratings yet
- Practice TestDocument14 pagesPractice TestHimanshu JindalNo ratings yet
- (02-12-14) AlkenesDocument4 pages(02-12-14) Alkenessasi.curieNo ratings yet
- Review Test 6 SolDocument14 pagesReview Test 6 SolVenkatakrishnan ANo ratings yet
- Function & Inverse-3 PDFDocument2 pagesFunction & Inverse-3 PDFVASU JAINNo ratings yet
- FIITJEE - JEE (Main) 1Document14 pagesFIITJEE - JEE (Main) 1Aditya Jain100% (1)
- Class Test MechanicsDocument10 pagesClass Test MechanicsTushif RahmanNo ratings yet
- Target: Jee (Advanced) 2019: P H Y S I C SDocument8 pagesTarget: Jee (Advanced) 2019: P H Y S I C SShreyansh SaxenaNo ratings yet
- Champ Square Term 4 Paper AdvanceDocument13 pagesChamp Square Term 4 Paper Advancepoonam guptaNo ratings yet
- M A T H E M A T I C S: DeterminantDocument11 pagesM A T H E M A T I C S: DeterminantJainNo ratings yet
- (3924) DPPDocument157 pages(3924) DPPBeyond ur imaginationNo ratings yet
- Goc FinalsheetDocument49 pagesGoc FinalsheetKartik KambleNo ratings yet
- Bansal Test Solidstate PDFDocument10 pagesBansal Test Solidstate PDFTarun Gupta100% (1)
- Test, Bansal Chemicalequilibrium PDFDocument18 pagesTest, Bansal Chemicalequilibrium PDFTarun Gupta0% (2)
- JEE Advanced 2014 Question Paper Solutions by Aakash InstituteDocument29 pagesJEE Advanced 2014 Question Paper Solutions by Aakash InstituteAnweshaBoseNo ratings yet
- Function & Inverse-1 PDFDocument3 pagesFunction & Inverse-1 PDFVASU JAINNo ratings yet
- Application of Derivative: Question Bank ONDocument20 pagesApplication of Derivative: Question Bank ONanubhav kumarNo ratings yet
- MATHS SECTION SINGLE CHOICE PARABOLAS FOCUS DIRECTRIXDocument2 pagesMATHS SECTION SINGLE CHOICE PARABOLAS FOCUS DIRECTRIXRahul JainNo ratings yet
- FIITJEE KVPY MOCK TEST-1 CLASS 11 A LOTDocument7 pagesFIITJEE KVPY MOCK TEST-1 CLASS 11 A LOTTEJA SINGH0% (2)
- Application of DerivativeDocument36 pagesApplication of DerivativeRaju SinghNo ratings yet
- Road Map (3) Problem: Aq. KOHDocument1 pageRoad Map (3) Problem: Aq. KOHShubham RajNo ratings yet
- Best Approach: Compound AngleDocument8 pagesBest Approach: Compound AngleAbhiyanshu KumarNo ratings yet
- Super Sixer 6 IsomerismDocument4 pagesSuper Sixer 6 IsomerismKartik YadavNo ratings yet
- 09.07.2023 - Enthu SRG - Phase 1 & 2 - (Adv) - It-4Document24 pages09.07.2023 - Enthu SRG - Phase 1 & 2 - (Adv) - It-4Priyansh BNo ratings yet
- Test - A: BR (1) CH BR (2) (4) BRH C - H CDocument5 pagesTest - A: BR (1) CH BR (2) (4) BRH C - H CVansh ChauhanNo ratings yet
- 38 Class Test (N-TOPS, TNPS) - SolutionDocument11 pages38 Class Test (N-TOPS, TNPS) - Solutionshivam bharti100% (1)
- Mock Test 8 Paper 2 Question PDFDocument26 pagesMock Test 8 Paper 2 Question PDFSidNo ratings yet
- Aakash ITtor 1Document37 pagesAakash ITtor 1Sujit Laware100% (2)
- Periodic Properties: Exercise-1Document41 pagesPeriodic Properties: Exercise-1K.S.MAYILVAGHANANNo ratings yet
- M A T H e M A T I C SDocument5 pagesM A T H e M A T I C SAtharva GanjuNo ratings yet
- Coordination Compound: Inorganic ChemistryDocument55 pagesCoordination Compound: Inorganic ChemistrySaanvi JoshiNo ratings yet
- Maths DPPDocument4 pagesMaths DPPAshish RanjanNo ratings yet
- LPP-Fluid Mechanics: FiitjeeDocument5 pagesLPP-Fluid Mechanics: FiitjeeYash TandonNo ratings yet
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- Illuminati - 2020: Advanced Physics Assignment-3Document25 pagesIlluminati - 2020: Advanced Physics Assignment-3Yash DhokeNo ratings yet
- Alkynes and RadicalsDocument7 pagesAlkynes and RadicalsArmando Shehi SayhellotogoodbyeNo ratings yet
- Program Chem Check-Up 2.0: Predicting structures, naming compounds and identifying reactionsDocument2 pagesProgram Chem Check-Up 2.0: Predicting structures, naming compounds and identifying reactionsanis fazilaNo ratings yet
- Chemistry Paper - Ii Solution (Code 3)Document5 pagesChemistry Paper - Ii Solution (Code 3)kolodoloNo ratings yet
- Electric Flux, Gauss's Law and Its ApplicationsDocument2 pagesElectric Flux, Gauss's Law and Its ApplicationsAariya KumariNo ratings yet
- Jharkhand Bijli Vitran Nigam Limited: Energy BillDocument2 pagesJharkhand Bijli Vitran Nigam Limited: Energy BillAariya KumariNo ratings yet
- ELECTRIC FLUX & GAUSS'S LAW - QuizDocument5 pagesELECTRIC FLUX & GAUSS'S LAW - QuizAariya KumariNo ratings yet
- Electric Potential Practice ProblemsDocument2 pagesElectric Potential Practice ProblemsAariya KumariNo ratings yet
- Hydrocarbon: Target Iit Jee 2017 Xii (VS+VR)Document36 pagesHydrocarbon: Target Iit Jee 2017 Xii (VS+VR)Aariya KumariNo ratings yet
- Don't Touch Its Mine - AariyaDocument1 pageDon't Touch Its Mine - AariyaAariya KumariNo ratings yet
- AldehydeDocument29 pagesAldehydeJan michael ChivaNo ratings yet
- Chapter 20: Carboxylic Acids and Nitriles: Please ReadDocument12 pagesChapter 20: Carboxylic Acids and Nitriles: Please ReadNeil GaymanNo ratings yet
- Conrad-Limpach Quinoline Synthesis: A. General Description of The ReactionDocument5 pagesConrad-Limpach Quinoline Synthesis: A. General Description of The ReactionLe Tu0% (1)
- Aldol Condensation DiscussionDocument3 pagesAldol Condensation DiscussionDenisse Watt Cuarteros100% (8)
- AlkaloidDocument10 pagesAlkaloidAde Rizki AnggrainiNo ratings yet
- Spectroscopic Solutions of StructureDocument21 pagesSpectroscopic Solutions of StructureKassimNo ratings yet
- Alkyl Halides & Aryl Halides: Victor GrignardDocument50 pagesAlkyl Halides & Aryl Halides: Victor GrignardsarahNo ratings yet
- Pmoc Lab ReviewerDocument6 pagesPmoc Lab ReviewerJasper JangNo ratings yet
- Basic Organic Nomenclature Packet Honors Chemistry: Name: - BlockDocument12 pagesBasic Organic Nomenclature Packet Honors Chemistry: Name: - BlockJamaica Calamno SalvadorNo ratings yet
- Chitosan Catalyzed Synthesis of IminesDocument6 pagesChitosan Catalyzed Synthesis of IminesaustingoewertNo ratings yet
- CHE 232-001 Organic Chemistry Exam 3 April 3, 1996: Name Student ID NoDocument8 pagesCHE 232-001 Organic Chemistry Exam 3 April 3, 1996: Name Student ID NoVinh HoangNo ratings yet
- Perception Test 4 Organic Chem Sem 2Document5 pagesPerception Test 4 Organic Chem Sem 2gypsydavesbrotherNo ratings yet
- Articulo 10.-Boric Acid As A Green Catalyst For The Conversion of Aldehydes and KetonesDocument8 pagesArticulo 10.-Boric Acid As A Green Catalyst For The Conversion of Aldehydes and KetonesPaul Delgado MendozaNo ratings yet
- Functional GroupCH5Document36 pagesFunctional GroupCH5syedmcgarretNo ratings yet
- Common, Possible Patterns of ResonanceDocument13 pagesCommon, Possible Patterns of ResonanceFarhana Mohd RazaliNo ratings yet
- Pd Complex Reactions With Unsaturated LigandsDocument81 pagesPd Complex Reactions With Unsaturated LigandschristopheNo ratings yet
- Chapter 10 Wade 7th - 2Document27 pagesChapter 10 Wade 7th - 2Afra FitrianitaNo ratings yet
- A Review On Recent Developments and Progress in The Kinetics Anddeactivation of Catalytic Acetylation of Glycerol-A Byproduct of BiodieselDocument15 pagesA Review On Recent Developments and Progress in The Kinetics Anddeactivation of Catalytic Acetylation of Glycerol-A Byproduct of BiodieselanisaNo ratings yet
- Aldol Condensation and Synthesis of DibenzalacetoneDocument8 pagesAldol Condensation and Synthesis of DibenzalacetoneArturo CamañoNo ratings yet
- Classification Tests For Hydroxyl - and Carbonyl - Containing CompoundsDocument6 pagesClassification Tests For Hydroxyl - and Carbonyl - Containing CompoundsShaira Jhann L. Rosales50% (2)
- Telangana TS EdCET Syllabus and Exam PatternDocument43 pagesTelangana TS EdCET Syllabus and Exam PatternpavaniNo ratings yet
- IR Absorption Table PDFDocument3 pagesIR Absorption Table PDFDavid QuinteroNo ratings yet
- COM123Document44 pagesCOM123Mùbãrâk MøhàmmãdNo ratings yet
- Schiff and Mannich ReactionsDocument16 pagesSchiff and Mannich ReactionsSat MontesNo ratings yet
- Pharmacy SyllabusDocument89 pagesPharmacy SyllabusDivvela ManoharNo ratings yet
- The Grignard Reaction Unraveling A Chemical PuzzleDocument11 pagesThe Grignard Reaction Unraveling A Chemical PuzzleAvinashNo ratings yet
- Asam Karboksilat Dan TurunannyaDocument93 pagesAsam Karboksilat Dan TurunannyaUswah HasanahNo ratings yet
- Chemistry of Carbon Compounds 2nd Supplement To 2r.e v.3B, C, D by Malcolm SainsburyDocument383 pagesChemistry of Carbon Compounds 2nd Supplement To 2r.e v.3B, C, D by Malcolm SainsburyMarius Stelian RusNo ratings yet
- 11 Chemoselectivity (Part 1+2 Redox)Document35 pages11 Chemoselectivity (Part 1+2 Redox)barry allenNo ratings yet
- ch17 SummaryDocument1 pagech17 Summaryapi-465421809No ratings yet