Professional Documents
Culture Documents
ch17 Summary
Uploaded by
api-465421809Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ch17 Summary
Uploaded by
api-465421809Copyright:
Available Formats
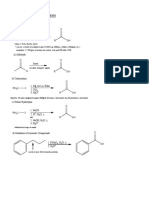
Summary of CH17 Aldehydes and Ketones • Ketone/aldehyde protection as cyclic thioacetal (see VI above): stable to
I. Nomenclature base and H3O+, LiAlH4, RLi, RMgX, etc. (complementary to cyclic acetals)
• Common names • IUPAC name • vic-diol protection as cyclic acetals: stable to base, LiAlH4, RLi, RMgX, etc
II. Synthesis of aldehydes and ketones HO OH H+ R' O CH3
• Oxidation of alcohols (review section 8.6) CH3COCH3
R O CH3
R' R
PCC, CH2Cl2: 1° RCH2OH ––>RCHO
MnO2: allylic or benzylic C=CH–CH2OH ––> C=CH–CHO VIII. Addition of H-NH2 and its derivatives to form imines,
Jones reagent: 2° RR'CHOH ––>RR'C=O enamines, oximes, hydrazones, semicarbazones
• All additions are under acid catalysis.
• Ozonolysis of alkenes (review section 12.11) • Addition of H-HN2 or H-NHR (1° amine) to C=O forms imine
R1R2C=CHR3 ––>R1R2C=O (ketones) + R3CHO (aldehyde) R'
R' H+
• Hydration of alkynes (review section 13.8 & 13.9) R' = alkyl (ketone)
O H–NHR NR
R' = H (aldehyde)
(A) Markovnikov hydration to ketones, (B) anti-Markovnikov hydration to R R
aldehydes • Addition of H-NR1R2 (2° amine) to C=O containing -H forms enamine
1. R2BH
Hg2+, H2SO4, H2O O (B)R C CH RCH2 CHO
(A)R C CH 2. H2O2, HO– R' = alkyl (ketone) R' H+ R'
R CH3
R' = H (aldehyde) O H–NR2 NR''2
•Friedel-Crafts acylation (review section 15.14)
RCH2 RHC
O O
• Additions of H-NHOH, H-NHNHR, or NH2NHCONH2, H+form crystaline
CO, HCl, AlCl3 RCOCl, AlCl3
H R compounds that are often used in identifying unknown aldehydes or ketones
H–NHOH, H+ R'
S S S phenones R'
benzaldehydes N-OH oxime
III. General mechanisms for nucleophilic addition to C=O O R
R R'
• Nucleophilic addition-protonation (if Nu is a strong nucleophile
R' = alkyl (ketone) NH2NHR, H+ NNHR
and the reaction is under basic conditions)
R' = H (aldehyde) R hydrazone
H 2O R'
– O –
C O Nu OH
N-NHCONH 2
Nu Nu
+ R semicarbazone
NH2NHCONH 2, H
• Electrophilic protonation-addition (if Nu is a weak nucleophile and the
reaction is under acidic conditions) • Wolff-Kishner reduction: to reduce C=O to -CH2- under strong basic
H+ conditions
Nu– R' NH2NH2, NaOH, heat H
C O C OH OH R' = alkyl (ketone) R'
O C H
Nu R' = H (aldehyde) R
R
IV. Addition of H-OH to form hydrates Compare the C=O to –CH2-reduction by Wolf-Kishner reduction,
• Acid or base catalysis
Clemmensen reduction, or thioacetal-hydrogenolysis reactions and
• Reversible reaction with the equilibrium favoring aldehyde or ketone
R' their respective limitations.
R H+ or HO–
R' = alkyl (ketone) R IX. Addition of H-CN to form cyanohydrins
C O H 2O OH
R' = H (aldehyde) • Acid-catalyzed addition
R' HO hydrate • Cyanohydrins are versatile synthetic intermediates
V. Addition of H–OR to form acetals HCl RH2C – hydroxy acid
• Acid or base catalysis R'
O RH 2 C CO 2H
• Reversible reaction with the equilibrium favoring aldehyde or ketone H+ HO
R' R'
R H+ or HO– R' CN conc. H2SO4 –unsaturated
R' = alkyl (ketone) R R' CH2R RHC
C O O R'' OH HO acid
R' = H (aldehyde) CO2H
R' H R''O cyanohydrin
hemiacetal H–CN 1. LiAlH4 RH2C
R' –hydroxy
• 5– or 6– membered cyclic hemiacetals are favored and relatively stable 2. H2O CH2NH2 amine
R' = alkyl (ketone)
R H+ or HO– OH HO
cyclic X. Addition of phosphorus ylides: The Wittig reaction
R' = H (aldehyde) OH O O R hemiacetal
• Preparation of phosphorus ylides
• Hemiacetals are further converted into acetals under acidic conditions 1. P(C6H5)3, benzene RHC P(C6H5)3
RCH2X
R' H , + R' (1°, 2° alkyl halide)
2. strong base (ylide)
R O R'' R (RLi, NaH, or RONa/ROH)
hemiacetal OH OR''
R''O H • Synthesis of alkene from C=O compounds
R''O acetal
VI. Addition of H–SR to form thioacetals R' = alkyl (ketone) R' RHC P(C6H5)3 / THF R'
R' = H (aldehyde) O C RHC
• Thioacetal formation requires a Lewis acid catalyst such as BF3, or ZnCl2 R R
• Thioacetals are stable in H3O+ but can be hydrolyzed with a Lewis acid as XI. Addition of H–OOCOR: The Baeyer–Villiger oxidation
catalyst R BF3 or ZnCl2 R'
• Conversion of aldehydes (R'=H) to acids and ketones (R'=alkyl) to esters
R' = alkyl (ketone) R SR''
O
C O + 2 RSH O
R' = H (aldehyde) O
HgCl2, H2O, R''S thioacetal The migratory aptitude of R:
R' C R O OH, CH2Cl2
CH3CN C H > 3° > 2° > 1° > CH3
R' R
• Thioacetal formation - hydrogenolysis provides a method to reduce R' OR
C=O to -CH2– O HS XII. Oxidative chemical tests for aldehydes
Lewis acids S S
R' = alkyl (ketone) C H H • Fehling's test
R' = H (aldehyde) R' Raney Ni, H2
R R Cu2O (s)
R HS R' R Cu+, NaOH, H2O
R C O C O (brick red)
R'
VII. Acetals and thioacetals as protecting groups in synthesis H HO
• ketone/aldehyde protection as cyclic acetal: stable to base, LiAlH4, RLi, • Tollen's test
RMgX HO +
O H O O
R' = alkyl (ketone) Ag+
O O Ag (s)
R' = H (aldehyde) C cyclic acetal C NH3, H2O C (mirror)
R' R HO R' R R H R OH
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Named Reactions: 6.1. Aldol CondensationDocument17 pagesNamed Reactions: 6.1. Aldol CondensationNikunja samalNo ratings yet
- Organic Reducing Agents ListDocument10 pagesOrganic Reducing Agents ListJatin BhasinNo ratings yet
- Amino Acid and BiochemistryDocument10 pagesAmino Acid and BiochemistryUNKNOWNNo ratings yet
- Alcohols: Nomenclature Properties Preparation Reactions SpectrosDocument38 pagesAlcohols: Nomenclature Properties Preparation Reactions SpectrosjuvyneilNo ratings yet
- Chapter 8 Reactions of AlcoholsDocument12 pagesChapter 8 Reactions of AlcoholsRoberto SIlvaNo ratings yet
- Chapter 21 NotesDocument43 pagesChapter 21 NotesTiffany YehNo ratings yet
- Alkynes Medorg2Document6 pagesAlkynes Medorg2AR LazagaNo ratings yet
- Carboxylic AcidsDocument26 pagesCarboxylic Acidsapi-3734333100% (1)
- Heterocyclic Chemistry: Chapter 10:pyrroles, Reactions and SynthesisDocument30 pagesHeterocyclic Chemistry: Chapter 10:pyrroles, Reactions and SynthesisTaciturnoait NihilistaNo ratings yet
- Organic Chemistry New Gyan SutraDocument8 pagesOrganic Chemistry New Gyan SutraSatyam JaiswalNo ratings yet
- Mind Map (Hydrocarbons)Document3 pagesMind Map (Hydrocarbons)Meenakshi NairNo ratings yet
- Aldehydes and Ketones: Key Reactions and MechanismsDocument7 pagesAldehydes and Ketones: Key Reactions and MechanismsA LEVEL TOPNo ratings yet
- Carboxylic Acids and Their Derivatives ExplainedDocument26 pagesCarboxylic Acids and Their Derivatives ExplainedAyush Gangwani50% (2)
- Reactions of Aldehydes, Ketones, and Carboxylic AcidsDocument3 pagesReactions of Aldehydes, Ketones, and Carboxylic AcidsErica TepepaNo ratings yet
- Reactive Intermediates: Arynes, Carbenes, and NitrenesDocument115 pagesReactive Intermediates: Arynes, Carbenes, and NitrenesMuhammad ArsalanNo ratings yet
- BeckmannDocument17 pagesBeckmannMaryam KhushbakhatNo ratings yet
- Hydrocarbon: Ashwani Tyagi Sir (Code: ATJEE)Document22 pagesHydrocarbon: Ashwani Tyagi Sir (Code: ATJEE)Prince DigvijayNo ratings yet
- Chap 16 Aldehydes and KetonesDocument88 pagesChap 16 Aldehydes and KetonesAna Liza DolomandingNo ratings yet
- Functional Group Transformation Using Sn2 ReactionDocument13 pagesFunctional Group Transformation Using Sn2 Reactionkurniatriwijaya.2410No ratings yet
- Organic Reactions and Functional Group SynthesisDocument48 pagesOrganic Reactions and Functional Group SynthesisRonak MantriNo ratings yet
- Road Map Organic PDFDocument5 pagesRoad Map Organic PDFS SquareNo ratings yet
- 5L ReductionsDocument20 pages5L ReductionsCarlos Javier Orellana OrtizNo ratings yet
- Winstein: Concept of ion pairs and carbocation stabilityDocument14 pagesWinstein: Concept of ion pairs and carbocation stabilityAnil KumarNo ratings yet
- Hydrocarbon 13 THDocument20 pagesHydrocarbon 13 THRaju SinghNo ratings yet
- Alkane: General Methods of Preparation: (1) by Catalytic Reduction of Alkenes and AlkynesDocument11 pagesAlkane: General Methods of Preparation: (1) by Catalytic Reduction of Alkenes and AlkynesaashishNo ratings yet
- HydrocarbonDocument94 pagesHydrocarbonArshNo ratings yet
- GMP GR: Reaction Chart For AlkanesDocument3 pagesGMP GR: Reaction Chart For AlkanesManoj DesaiNo ratings yet
- Hydrocarbon (12th)Document22 pagesHydrocarbon (12th)Raju SinghNo ratings yet
- NMRshifts1H GeneralDocument1 pageNMRshifts1H GeneralJeric CestinaNo ratings yet
- NPTEL Organometallic Reagents Grignard Reactions Carbonyl AdditionDocument130 pagesNPTEL Organometallic Reagents Grignard Reactions Carbonyl Additionaneeda shabirNo ratings yet
- Aldehydes and KetonesDocument19 pagesAldehydes and KetonesVaibhav TarkasbandNo ratings yet
- Haloalkanes and HaloarenesDocument26 pagesHaloalkanes and Haloarenesrajputrishi1982No ratings yet
- Topic 16 Aldehydes, Ketones and Optical Isomerism Reactions of Aldehydes and Ketones Optical IsomerismDocument15 pagesTopic 16 Aldehydes, Ketones and Optical Isomerism Reactions of Aldehydes and Ketones Optical Isomerismclip215No ratings yet
- Aldehydes + Ketones - Lecture IDocument41 pagesAldehydes + Ketones - Lecture IVanessa Osafo MensahNo ratings yet
- Nucleophilic Addition To The Carbonyl GroupDocument16 pagesNucleophilic Addition To The Carbonyl GroupYuni PurnamasariNo ratings yet
- Table of K ValuesDocument7 pagesTable of K ValuesdasoodaseeNo ratings yet
- Kap 12,13,17, IIDocument12 pagesKap 12,13,17, IImuraliNo ratings yet
- IIT-JEE Chemistry by N.J. sir: Aldehydes and KetonesDocument10 pagesIIT-JEE Chemistry by N.J. sir: Aldehydes and KetonesMahendra ChouhanNo ratings yet
- Roadmap - Main Corrected 2013Document1 pageRoadmap - Main Corrected 2013Luân Chu Nguyễn NhậtNo ratings yet
- Reactions and Interconversions of Organic Functional GroupsDocument3 pagesReactions and Interconversions of Organic Functional Groupsmichelsonyip100% (1)
- CHEM 360 NOTES: CARBOXYLIC ACIDS AND ESTERSDocument36 pagesCHEM 360 NOTES: CARBOXYLIC ACIDS AND ESTERSThục NghiNo ratings yet
- Chapter 8 SlidesDocument63 pagesChapter 8 SlidespoojaNo ratings yet
- Hydrocarbon: Target Iit Jee 2017 Xii (VS+VR)Document36 pagesHydrocarbon: Target Iit Jee 2017 Xii (VS+VR)Aariya KumariNo ratings yet
- 13. AminesDocument24 pages13. AminesRajdeep Singh RahiNo ratings yet
- ReductionDocument7 pagesReductionPranayNo ratings yet
- Organic Chmeistry Chapter 10Document9 pagesOrganic Chmeistry Chapter 10yoojh100No ratings yet
- Organic Synthesis. ReductionsDocument64 pagesOrganic Synthesis. ReductionsKartik RanaNo ratings yet
- Alkane: Preparation of Alkanes (6-Methods)Document20 pagesAlkane: Preparation of Alkanes (6-Methods)siddanshNo ratings yet
- Reductions by lithium aluminium hydride (LiAlH4Document15 pagesReductions by lithium aluminium hydride (LiAlH4pranshul jadonNo ratings yet
- Organic Synthesis Via Enolates BSC III CH IVDocument10 pagesOrganic Synthesis Via Enolates BSC III CH IVSanjay ShirodkarNo ratings yet
- Ester RxnsDocument1 pageEster Rxnsapi-465421809No ratings yet
- Aldehydes Ketones and Acids ClassifiedDocument15 pagesAldehydes Ketones and Acids ClassifiedSsNo ratings yet
- 1 Theory2Document16 pages1 Theory2Tushar RajNo ratings yet
- Substitution Elimination (Alkilhalides) Bab 5 FessendenDocument113 pagesSubstitution Elimination (Alkilhalides) Bab 5 Fessendenahmad jamalNo ratings yet
- Chem12 C2300 SWBTDocument15 pagesChem12 C2300 SWBTAbdulrahman MaherNo ratings yet
- Chem12 C2300 SWBTDocument15 pagesChem12 C2300 SWBTAbdulrahman MaherNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsFrom EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsNo ratings yet
- Ca RXN CheckDocument1 pageCa RXN Checkapi-4654218090% (1)
- Ca SynthDocument1 pageCa Synthapi-465421809No ratings yet
- Phenol SynthDocument1 pagePhenol Synthapi-465421809No ratings yet
- 118c Practice Synthesis KeyDocument18 pages118c Practice Synthesis Keyapi-465421809No ratings yet
- 118c Practice Synthesis KeyDocument18 pages118c Practice Synthesis Keyapi-465421809No ratings yet
- Benzene RxnsDocument1 pageBenzene Rxnsapi-465421809No ratings yet
- Phenol Rxns IDocument1 pagePhenol Rxns Iapi-465421809No ratings yet
- Ester RxnsDocument1 pageEster Rxnsapi-465421809No ratings yet
- Ca RXN CheckDocument1 pageCa RXN Checkapi-4654218090% (1)
- Anhydride RxnsDocument1 pageAnhydride Rxnsapi-465421809No ratings yet
- Ca Rxns IIDocument1 pageCa Rxns IIapi-465421809No ratings yet
- Acyl Halide RxnsDocument1 pageAcyl Halide Rxnsapi-465421809No ratings yet
- Amide RxnsDocument1 pageAmide Rxnsapi-465421809No ratings yet
- Amine SynthDocument1 pageAmine Synthapi-465421809No ratings yet
- Week 6 Practice Final Part 2 Chapter 18Document4 pagesWeek 6 Practice Final Part 2 Chapter 18api-465421809No ratings yet
- Nitrile RxnsDocument1 pageNitrile Rxnsapi-465421809No ratings yet
- Ca SynthDocument1 pageCa Synthapi-465421809No ratings yet
- Week 5 Practice Final Part 1 - New Material 1Document3 pagesWeek 5 Practice Final Part 1 - New Material 1api-465421809No ratings yet
- 118b mt1 Study Guide 2Document21 pages118b mt1 Study Guide 2api-465421809No ratings yet
- Ca Rxns IDocument1 pageCa Rxns Iapi-465421809No ratings yet
- CH 17: Aldehydes and Ketones: Nasiri CHE118B Final Study Guide Spring 2019 - S. Ly Sdly@ucdavis - EduDocument17 pagesCH 17: Aldehydes and Ketones: Nasiri CHE118B Final Study Guide Spring 2019 - S. Ly Sdly@ucdavis - Eduapi-465421809No ratings yet
- Midterm 1 Practice QuestionsDocument8 pagesMidterm 1 Practice Questionsapi-465421809No ratings yet
- 118 BprsynthDocument19 pages118 Bprsynthapi-465421809No ratings yet
- ch18 SummaryDocument1 pagech18 Summaryapi-465421809No ratings yet
- MOC Alcohol RXN Map PDFDocument2 pagesMOC Alcohol RXN Map PDFNickOoPandeyNo ratings yet
- ch15-16 SummaryDocument1 pagech15-16 Summaryapi-465421809No ratings yet
- 118b mt2 Study Guide 2Document20 pages118b mt2 Study Guide 2api-465421809No ratings yet
- ch14 SummaryDocument1 pagech14 Summaryapi-465421809No ratings yet
- Resonance Practice ProblemsDocument12 pagesResonance Practice Problemsapi-465421809No ratings yet
- Problema Sherlock Holmes PDFDocument53 pagesProblema Sherlock Holmes PDFLeonardo EmilianoNo ratings yet
- Reaction Kinetics ExplainedDocument31 pagesReaction Kinetics ExplainedchweetomahiNo ratings yet
- Biochemistry PDFDocument15 pagesBiochemistry PDFJaz SantosNo ratings yet
- CHM 152 Final Exam Review 1 Spring 2012 NEW KEYDocument4 pagesCHM 152 Final Exam Review 1 Spring 2012 NEW KEYCaguioa Mark Anthony G.No ratings yet
- Chapter 16 Study SlidesDocument35 pagesChapter 16 Study SlidesMakenzie DownsNo ratings yet
- Chemical Reactor Design Chemical Reactor Design: Y W L Youn-Woo LeeDocument41 pagesChemical Reactor Design Chemical Reactor Design: Y W L Youn-Woo LeePara DiseNo ratings yet
- Reaction MechanismDocument37 pagesReaction MechanismNurshuhada NordinNo ratings yet
- MCD4390 Week 10 Tutorial QuestionsDocument5 pagesMCD4390 Week 10 Tutorial QuestionsGabbar100% (1)
- Pericyclic Reactions OverviewDocument89 pagesPericyclic Reactions OverviewSandipan Saha100% (1)
- Anionic PolymerisationDocument3 pagesAnionic PolymerisationChayanAnandNo ratings yet
- Chemical Kinetics and Reactor Design EquationsDocument60 pagesChemical Kinetics and Reactor Design EquationsMaryjean Almodiel InfornonNo ratings yet
- NEET - Haloalkanes & Haloarenes - (Q+S)Document18 pagesNEET - Haloalkanes & Haloarenes - (Q+S)Sachin DedhiaNo ratings yet
- Reforming Catalyst Regenration: M.Saleem Chohan Syed Kashif HassanDocument17 pagesReforming Catalyst Regenration: M.Saleem Chohan Syed Kashif HassanSaleem ChohanNo ratings yet
- Alkene - Alkynes 1Document39 pagesAlkene - Alkynes 1Hajar MuhamadNo ratings yet
- ch13 ProblemsDocument84 pagesch13 Problemsbrownhazel67% (6)
- Chemical Reaction Engineering10 - 2010Document33 pagesChemical Reaction Engineering10 - 2010Ingrid Claudia ElianneNo ratings yet
- Updated Advanced EnzymologyDocument16 pagesUpdated Advanced Enzymologybrandon899williamsNo ratings yet
- Reaction MechanismDocument41 pagesReaction MechanismJyöt SîlvērNo ratings yet
- KOT122 Course IntroductionDocument5 pagesKOT122 Course IntroductionFarihah FazimNo ratings yet
- IGCSE Student Revision Power Point Topic 5 - 複本Document16 pagesIGCSE Student Revision Power Point Topic 5 - 複本yt kNo ratings yet
- Elimination Reactions Mechanism Lecture NotesDocument17 pagesElimination Reactions Mechanism Lecture NotesveluselvamaniNo ratings yet
- Name: Kumar Kartikey Agarwal: Experiment 1: Isothermal Batch ReactorDocument6 pagesName: Kumar Kartikey Agarwal: Experiment 1: Isothermal Batch ReactorKartikey AgarwalNo ratings yet
- CH 15Document145 pagesCH 15esin buzNo ratings yet
- Chapter 7Document30 pagesChapter 7Apichat Junsod100% (4)
- Chemical Kinetics Rate LawsDocument35 pagesChemical Kinetics Rate LawsRichie SuyaoNo ratings yet
- 5TH FORM CHEMISTRY - POLYMER WORKSHEET ON GLUCOSE, STARCH AND PROTEINSDocument4 pages5TH FORM CHEMISTRY - POLYMER WORKSHEET ON GLUCOSE, STARCH AND PROTEINSZantaye Thomas100% (1)
- Chemical KineticsDocument40 pagesChemical KineticsHirdesh Sehgal100% (3)
- Synthesis and Recrystallization of Dibenzalacetone: Experiment - 4Document2 pagesSynthesis and Recrystallization of Dibenzalacetone: Experiment - 4ARYAN CHAVANNo ratings yet
- REACTOR ISOMERIZATION OF LIGHT NAPHTHADocument8 pagesREACTOR ISOMERIZATION OF LIGHT NAPHTHAاحمد حمید کارسول عزیزNo ratings yet
- CHNG 3004 - 2019-2020 AssignmentsDocument26 pagesCHNG 3004 - 2019-2020 AssignmentsXheikhKaleem100% (1)