Professional Documents
Culture Documents
Solutions INChO 2008 PDF
Uploaded by
Divyansh JainOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Solutions INChO 2008 PDF
Uploaded by
Divyansh JainCopyright:
Available Formats
Answers to INChO-2008 problems
Question No. 1
Subdivisions
1.1) F2 -1.6, F2 - 1.1,1.3
1.2) F atom -3.4, F2 molecule - 3.0,3.2
1.3) F2: -1.4 F2: -1.9
1.4) 4.15
1.5)
2p
2p
2p 2p
2p
2p
2s
2s
F2
F2
1.6) F2 – 1.0 F2 - 0.5
1.7) F2
1.8) I1= 18.9 eV I2= 15.6 eV
1.9) I1= 2p I2 = 2p
HBCSE, 2nd February 2008 1
Question No. 2
Subdivisions
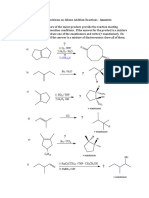
2.1) a) Z-5-methyl hex-2-en-1-al
b) Z-2-methyl-1-phenyl hept-1-en-6-yne
2.2) B C
T.S.1
T.S.2
2.3)
Free energy
Int
product
Reaction Coordinate
2.4) i) E
ii) D
iii) E
2.5) a) iv b) iii c) ii d) v e) i
H
2.6) O O
H
OO OO
H
O H O
H
CH2 C C CH3
2.7) D.
O
CH2COOH
COOH O
E. F.
O O
O
OH
OH
2.8) G. Br CH2 CH CH CH2 Br
Br CH2 CH CH CH2
H.
Br
HBCSE, 2nd February 2008 2
I. J.
CH2Br CH2Br
R R
H Br H Br
S R
H Br Br H
CH2Br CH2Br
O O O
2.9) O O
O
O O
K
O
2.10) O
L
O
Question No. 3
Subdivisions
3.1)
i) aromatic iv) yes
ii) aromatic v) acidic
iii) vi) a)
3.2) I < III < II
COOH COOH
3.3) K L
HO H7C3O
NO2 NO2
COCl OH
M N N(C2H5)2
H7C3O
NO2 O
O O C OC3H7
O C OC3H7 NH2
O P N(C2H5)2
N(C2H5)2 NO2
HBCSE, 2nd February 2008 3
Br NO2 NO2
3.4) Br
O O OMe
OMe
Br
+ Br Br
3.5) A. B. C. D.
+
N N
Br
3.6) iv.
Question No. 4
Subdivisions
4.1) 8.17 10-8 bar
4.2) 304 K
4.3) 641.02 K
4.4) Yes the reaction will proceed towards NOCl
4.5) Rate = k [NO]2 [Cl2]
4.6) Ea = 98.82 kJ / mol
4.7) Mechanism I and II both are possible.
4.8) Extent of the reaction = 0.1
4.9) Extent of the reaction at completion = 0. 195
Question No. 5
Subdivisions
5.1) a.
5.2) b.
5.3) X1 Solid phase
X2 Solid –Liquid equilibrium phase
X3 Liquid – Gas equilibrium phase
X4 Gas Phase
HBCSE, 2nd February 2008 4
5.4)
gas
Liquid –gas
Temp
T0
equilibrium
Liquid
Tm Solid-liquid
equilibrium
solid
Time
5.5) b
5.6) Volume will increase on melting
5.7) Single Phase system called Supercritical Fluid
5.8) c
p1 1 p1 1 2 p1
5.9)
p1 p1 2 p1 or
p1 2 p1
p1 / p1 2
5.10) 56
5.11) a. nA =An
[ A] n KC 2
b.
C1
KD
n C2
HBCSE, 2nd February 2008 5
Question No. 6
Subdivisions
P
6.1)
P P
6.2) P4 (s) + 3 NaOH + 3H2O PH3(g) + 3 H2PO2 Na+
2
6.3) a. H + H H H
Na
or
P O or H P O P P
2 Na+
O O O ONa O ONa
H O
ONa
b. hypophosphite-reducing agent
phosphate –reducing agent
c. They are reducing agents due to presence of P-H bond and lower oxidation state of P
6.4) Ca5(PO4)3F + 5H2SO4 3H3PO4 + 5CaSO4 + HF
6.5) O
O
P P
O O
O O O O
P
or O P O P O
O O
O P O O
6.6) 504O gs Pof CaO.
O P
O
6.7) PCl3 + O2 2Cl3PO O
POCl3 + 3 EtOH O P(OEt)3 + 3 HCl
6.8)
3s 3p 3d
Ground
state
Excited
State
Cl
Cl
P Cl Or trigonal bipyramidal
Cl
Cl
HBCSE, 2nd February 2008 6
6.9) 3PCl5 + 3NH4Cl (Cl2PN)3 + 12 HCl
Cl Cl
P
N N
Cl Cl
P P
Cl Cl
N
HBCSE, 2nd February 2008 7
Question No. 7
Subdivisions
7.1) Co3+ : 3d6 4S0
7.2) A Pink: [Co(NH3)5.H2O]Cl3: Pentaamine aqua cobalt(III)chloride
B Purple: [CoCl(NH3)5]Cl2 : Pentaamine chlorocobalt(III)chloride
7.3) Co3+ = 3d6
3d 4s 4p
XX XX XX XX XX XX
d2sp3 hybridisation
Octahedral
7.4) a.
eg
t2g
3+ 6
Co =d
b. diamagnetic.
7.5) [Co(NH3)6]2+ = Co2+ [Co(NH3)6]3+ = Co3+
Loss of electron easy
HBCSE, 2nd February 2008 8
NH 3 NH3
7.6) NH 3 Cl
Cl Co NH 3 Cl Co NH3
Cl Cl
Cl NH3
Facial Meridional
7.7) Facial isomer - one peak due to ammonia (with all similar environments)
Meridional isomer – two peaks
7.8) Cl O
O O
O Co O Co
O Cl
O O
Cl Cl
Inactive plane of
symmetry Chiral
Question No. 8
Subdivisions
8.1) Fe(s) + 2OH(aq) FeO(s) + H2O(l) + 2e
Ni2O3 (s) + H2O(l) + 2e 2NiO(s) + 2OH(aq)
Fe(s) + Ni2O3 (s) FeO(s) + 2NiO(s)
8.2) iii.
8.3) E0 cell = 0.83633 V
Ecell = 0.80283 V
8.4) Ag(s) + Fe3+(aq) = Fe2+(aq) + Ag+(aq)
Fe(s) + 2Ag+(aq) = Fe2+(aq) + 2Ag(s)
8.5) K 2.97
[ Fe 3 ] 0.01
HBCSE, 2nd February 2008 9
Question No. 9
Subdivisions
9.1) H2NCH2COOH
9.2) i) Nylon COOH
ii) H2N CH2 NH2 + (CH2)6 COOH
9.3)
Nature Charge
Peptide
Acidic Basic Neutral Positive Negative Zero
Gly-Leu-Val X X
Leu-Trp-Lys-Gly-Lys X X
Arg-Ser-Val X X
+
9.4) a) H3N-CH-COO
CH2
CH2C6H5 CH2 CH3
+ + +
2 H3N-CH-COO + H3N-CH-COO + 2 H3N-CH-COO
b) +
H3N-CH-COO
SH
CH2
CH2
S (H) +
2 H3N-CH-COO
S
CH2
+
H3N-CH-COO
9.5) i) 4
H O COO
ii)
+
H3N C C NH C H
CH2COOCH3 CH2C6H5
HBCSE, 2nd February 2008 10
9.6) A – aspartic acid
B – alanine
C- arginine
HBCSE, 2nd February 2008 11
You might also like
- CH211 ALKYNES Problem Set 1. Provide The Products For TheDocument1 pageCH211 ALKYNES Problem Set 1. Provide The Products For ThekevinamyNo ratings yet
- Organic Chemistry: Key ConceptsDocument15 pagesOrganic Chemistry: Key ConceptsDhruv Jyot SinghNo ratings yet
- Final | Organic Chemistry 2Document1 pageFinal | Organic Chemistry 2Maryam AlaeiNo ratings yet
- Aziridinone Azetididone Buatanamide: Ome Pocl Reflux, 2HDocument2 pagesAziridinone Azetididone Buatanamide: Ome Pocl Reflux, 2HAllu HarikrishnaNo ratings yet
- Organic Chemistry 2 Homework 2: Vo Lam Hoai Trung BTCEIU19009Document4 pagesOrganic Chemistry 2 Homework 2: Vo Lam Hoai Trung BTCEIU19009Trung VõNo ratings yet
- 07 Addition Reactions 343 AnsDocument3 pages07 Addition Reactions 343 AnsPrince AbbeyNo ratings yet
- Chemistry I (Organic) : Stereochemistry: Diastereoisomers DR Alan Spivey Office: 834 C1 E-Mail: Tel.: 45841Document2 pagesChemistry I (Organic) : Stereochemistry: Diastereoisomers DR Alan Spivey Office: 834 C1 E-Mail: Tel.: 45841Francisco RomeroNo ratings yet
- Assignment 2 Sept2019-Jan2020Document4 pagesAssignment 2 Sept2019-Jan2020nanaNo ratings yet
- Carboxylic Acids & Derivatives: Chapter Practice ProblemsDocument3 pagesCarboxylic Acids & Derivatives: Chapter Practice ProblemsSushank MishraNo ratings yet
- Chemistry of Hetero AromaticsDocument139 pagesChemistry of Hetero Aromaticslalitarun100% (1)
- Indian Olympiad Qualifier in Chemistry (IOQC) 2020-2021Document9 pagesIndian Olympiad Qualifier in Chemistry (IOQC) 2020-2021Sankalp JainNo ratings yet
- Stereochemistry and Reaction MechanismsDocument2 pagesStereochemistry and Reaction MechanismsyemyemNo ratings yet
- Pract Prob Carboxylic Acids AnsDocument3 pagesPract Prob Carboxylic Acids AnsVictor HernandezNo ratings yet
- C H BR C H O (1S, 3S) - 3-MethylcyclohexanolDocument3 pagesC H BR C H O (1S, 3S) - 3-MethylcyclohexanolAnh Pham Le NgocNo ratings yet
- MIT5 111F14 ProbReviewDocument3 pagesMIT5 111F14 ProbReviewMD Abu RaselNo ratings yet
- Module12 PDFDocument30 pagesModule12 PDFVishalNo ratings yet
- 2007Document9 pages2007Anil KumarNo ratings yet
- Css ChemistryII 2017Document3 pagesCss ChemistryII 2017Bakhita MaryamNo ratings yet
- Chem 212 Condensation Reactions 3Document1 pageChem 212 Condensation Reactions 3kevinamyNo ratings yet
- Neet Ug 2023 Chemistry Paper With AnswerDocument7 pagesNeet Ug 2023 Chemistry Paper With Answervfg36579No ratings yet
- Class Test-2_Hydrocarbon (Hydrogenation)_Without AnswerDocument8 pagesClass Test-2_Hydrocarbon (Hydrogenation)_Without AnswerYuvarajNo ratings yet
- Photochemistry of Enones and DienonesDocument8 pagesPhotochemistry of Enones and DienonesSengottaiyan M MuruganNo ratings yet
- Madeleine Ceri - Final Exam CHE-A-02Document6 pagesMadeleine Ceri - Final Exam CHE-A-02Madeleine CeriNo ratings yet
- S20 2014 Exam2Document10 pagesS20 2014 Exam2laraNo ratings yet
- Alcohol, Phenol & EtheRDocument8 pagesAlcohol, Phenol & EtheRdevvratchoudhary11989No ratings yet
- CH CH Cooet Etooc: N H NH N NHDocument2 pagesCH CH Cooet Etooc: N H NH N NHAllu HarikrishnaNo ratings yet
- Solved Example: 1. The Final Product Obtained in The ReactionDocument43 pagesSolved Example: 1. The Final Product Obtained in The ReactionHardik SharmaNo ratings yet
- Short Answer Questions: Chem223 Practice Exam (Pre Final)Document2 pagesShort Answer Questions: Chem223 Practice Exam (Pre Final)Jenny WangNo ratings yet
- ExamF07 2Document3 pagesExamF07 2Anh Pham Le NgocNo ratings yet
- Activity: O H OH OH OH OH O HDocument3 pagesActivity: O H OH OH OH OH O HPercival GalahadNo ratings yet
- Chord ProgressionsDocument7 pagesChord ProgressionsdedejotaNo ratings yet
- Practice Exam 2CDocument10 pagesPractice Exam 2CĐình Thư LêNo ratings yet
- Organoboron and organosilicon chemistry tutorialDocument6 pagesOrganoboron and organosilicon chemistry tutorialBin RenNo ratings yet
- (+) - Lasonolide A (120414-TKGP) K. Shishido: ActivityDocument3 pages(+) - Lasonolide A (120414-TKGP) K. Shishido: ActivityPercival GalahadNo ratings yet
- Name: - CHEM 14D - Dr. Anish NagDocument5 pagesName: - CHEM 14D - Dr. Anish NagBob GooberNo ratings yet
- Incom. Sr. 29.06.2021 AssignmentDocument5 pagesIncom. Sr. 29.06.2021 AssignmentSrikar SatyaNo ratings yet
- Imperial College LondonDocument1 pageImperial College LondonCalum GlynnNo ratings yet
- 2012 Practice Exam 2-B: NameDocument10 pages2012 Practice Exam 2-B: NameĐình Thư LêNo ratings yet
- RearrangementsDocument82 pagesRearrangementsjoy shruthiNo ratings yet
- Organic Chemistry Assignment on Reactions & NomenclatureDocument6 pagesOrganic Chemistry Assignment on Reactions & NomenclatureAbdullah AlteneijiNo ratings yet
- Problems Seminar 2 - 2023 - 231004 - 144845Document7 pagesProblems Seminar 2 - 2023 - 231004 - 144845hectormunozroNo ratings yet
- Chem Academy: Enolate ChemistryDocument13 pagesChem Academy: Enolate ChemistryHamit RanaNo ratings yet
- IOQC2021 PartII Solutions 20210413Document9 pagesIOQC2021 PartII Solutions 20210413kritikasingh181221No ratings yet
- Alkyl HalidesDocument4 pagesAlkyl HalidesSantosh SharmaNo ratings yet
- Illustrations For Liber ChanokhDocument10 pagesIllustrations For Liber ChanokhCelephaïs Press / Unspeakable Press (Leng)100% (1)
- Organic Chemistry 2 (CHEM 30) For Bolero Final Exam PDFDocument15 pagesOrganic Chemistry 2 (CHEM 30) For Bolero Final Exam PDFKhangNo ratings yet
- Acid-base reactions, organic mechanisms, and stereochemistryDocument6 pagesAcid-base reactions, organic mechanisms, and stereochemistryhfweouNo ratings yet
- NQE 2009 Chemistry SolutionsDocument10 pagesNQE 2009 Chemistry Solutionsaleth felicianoNo ratings yet
- Exam Guide: 30 MCQs, 10 MSQs, 20 NATsDocument15 pagesExam Guide: 30 MCQs, 10 MSQs, 20 NATsAnil Kumar100% (1)
- Allen: Final Jee-Main Examination - July, 2021Document4 pagesAllen: Final Jee-Main Examination - July, 2021VEDANT JADHAONo ratings yet
- Frozen Solutions INChO 2011Document13 pagesFrozen Solutions INChO 2011Divyansh JainNo ratings yet
- Class Test-2_Hydrocarbon (Hydrogenation)_Without AnswerDocument8 pagesClass Test-2_Hydrocarbon (Hydrogenation)_Without AnswershouryatrialNo ratings yet
- JEE Advanced 2018 PaperDocument13 pagesJEE Advanced 2018 Papersaravanaajani2012No ratings yet
- MIT Problem Set #7: Acid-Base and Carbonyl ReactionsDocument5 pagesMIT Problem Set #7: Acid-Base and Carbonyl ReactionsKarthikeyanNo ratings yet
- Allen: Final Jee-Main Examination - February, 2021Document4 pagesAllen: Final Jee-Main Examination - February, 2021SajaNo ratings yet
- Alkyl Halide ReactionsDocument3 pagesAlkyl Halide ReactionsKryonNo ratings yet
- Exercise - VI (A) : IIT-JEE (Objective Problems)Document3 pagesExercise - VI (A) : IIT-JEE (Objective Problems)MoneyNo ratings yet
- e1e4b300ffc5fbe8f9d2830d555e0a4fDocument8 pagese1e4b300ffc5fbe8f9d2830d555e0a4fveenayaksachinsharmaNo ratings yet
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Bach for Guitar: 27 Transcriptions for GuitarFrom EverandBach for Guitar: 27 Transcriptions for GuitarRating: 5 out of 5 stars5/5 (10)
- Lelm103 PDFDocument11 pagesLelm103 PDFDivyansh JainNo ratings yet
- Indian National Physics Olympiad solutions and questionsDocument15 pagesIndian National Physics Olympiad solutions and questionsharshNo ratings yet
- Examination Syllabus For English Class Ix 2019-20: Delhi Public School SuratDocument2 pagesExamination Syllabus For English Class Ix 2019-20: Delhi Public School SuratDivyansh JainNo ratings yet
- NSE 2019 Chemistry Paper With Answer Rev PDFDocument17 pagesNSE 2019 Chemistry Paper With Answer Rev PDFaman pandeyNo ratings yet
- Frozen Solutions INChO 2011Document13 pagesFrozen Solutions INChO 2011Divyansh JainNo ratings yet
- Lelm302 PDFDocument13 pagesLelm302 PDFRitik ChaabraNo ratings yet
- Statewise Quota 2014Document1 pageStatewise Quota 2014PremMehtaNo ratings yet
- Ch10-Faraday's Law of InductionDocument32 pagesCh10-Faraday's Law of Inductionmehdii.heidary1366100% (3)
- Ss Exam Syllabus IxDocument1 pageSs Exam Syllabus IxDivyansh JainNo ratings yet
- KVPY 2015 Paper Solution SB - SX 2Document42 pagesKVPY 2015 Paper Solution SB - SX 2Anonymous Jrk1kd9NCNo ratings yet
- Examination Syllabus For English Class Ix 2019-20: Delhi Public School SuratDocument2 pagesExamination Syllabus For English Class Ix 2019-20: Delhi Public School SuratDivyansh JainNo ratings yet
- 2008 Sol PDFDocument11 pages2008 Sol PDFmadhav mittalNo ratings yet
- Nsec 2016 KeyDocument1 pageNsec 2016 KeySrinivas VenkataramanNo ratings yet
- Physical Chemistry For Joint Entrance ExaminatioDocument46 pagesPhysical Chemistry For Joint Entrance ExaminatioAnonymous tricksNo ratings yet
- ChemistryDocument3 pagesChemistryAnant SangtaniNo ratings yet
- Aits 1718 CRT II Jeem SolDocument20 pagesAits 1718 CRT II Jeem SolDivyansh JainNo ratings yet
- Examination Syllabus For Science Class Ix 2019-20: Delhi Public School SuratDocument1 pageExamination Syllabus For Science Class Ix 2019-20: Delhi Public School SuratDivyansh JainNo ratings yet
- Nsep 2014-15 Full EditionDocument24 pagesNsep 2014-15 Full EditionDivyansh JainNo ratings yet
- Indian Association of Physics Teachers: National Standard Examination in Chemistry 2017-2018 S E T - C 3 2 4 Answer KeyDocument1 pageIndian Association of Physics Teachers: National Standard Examination in Chemistry 2017-2018 S E T - C 3 2 4 Answer KeyDivyansh JainNo ratings yet
- INPhO2018 QuestionDocument28 pagesINPhO2018 Questionsaggnik sarkarNo ratings yet
- Paper KVPY XII Final Paper - 01!11!2015 - FinalDocument52 pagesPaper KVPY XII Final Paper - 01!11!2015 - FinalKartikeya AryaNo ratings yet
- Link:: QP Code P163 QP Code P160 QP Code P161 QP Code P162Document2 pagesLink:: QP Code P163 QP Code P160 QP Code P161 QP Code P162Divyansh JainNo ratings yet
- Paper KVPY XII Final Paper - 01!11!2015 - FinalDocument52 pagesPaper KVPY XII Final Paper - 01!11!2015 - FinalKartikeya AryaNo ratings yet
- Kvpy - Sa Stream - Weightage Analysis SheetDocument2 pagesKvpy - Sa Stream - Weightage Analysis SheetSai AkhilNo ratings yet
- Nsep 1516 Answer PDFDocument4 pagesNsep 1516 Answer PDFBabban SinghNo ratings yet
- Nsep Solved 2007Document10 pagesNsep Solved 2007Mahalingam NanjappanNo ratings yet
- Kvpy - Sa Stream - Weightage Analysis SheetDocument2 pagesKvpy - Sa Stream - Weightage Analysis SheetSai AkhilNo ratings yet
- Nsep 2014-15 Full EditionDocument24 pagesNsep 2014-15 Full EditionDivyansh JainNo ratings yet
- Mathematics KVPY Analysis SA 2007-2016Document1 pageMathematics KVPY Analysis SA 2007-2016Divyansh JainNo ratings yet
- Pancreatic Ductal Adenocarcinoma and Its Variants: Pearls and PerilsDocument20 pagesPancreatic Ductal Adenocarcinoma and Its Variants: Pearls and PerilsGeorge MogaNo ratings yet
- Ethnomusicology in Times of TroubleDocument15 pagesEthnomusicology in Times of TroubleLéo Corrêa BomfimNo ratings yet
- Sea Cliff Zanzibar E Fact SheetDocument6 pagesSea Cliff Zanzibar E Fact SheetBenedict MuringakumweNo ratings yet
- Service Manual: Side by Side S20B RSB21-A/GDocument16 pagesService Manual: Side by Side S20B RSB21-A/GjicutuNo ratings yet
- Sage Yb October2015Document460 pagesSage Yb October2015olopNo ratings yet
- Axell Wireless Cellular Coverage Solutions BrochureDocument8 pagesAxell Wireless Cellular Coverage Solutions BrochureBikash ShakyaNo ratings yet
- Drugs MnemonicsDocument6 pagesDrugs MnemonicsDarrylJavier100% (1)
- Lucy Mayienga CV RecentDocument3 pagesLucy Mayienga CV Recentlucy.mayiengaNo ratings yet
- Wire Rope Slings Si 2 - 2 EmmDocument2 pagesWire Rope Slings Si 2 - 2 EmmheppyfaebanffNo ratings yet
- Disease Causation 2Document32 pagesDisease Causation 2andualem werkinehNo ratings yet
- The Bone DreamingDocument3 pagesThe Bone DreamingastrozzNo ratings yet
- Standards.: General Fastener Standards BS OrderDocument33 pagesStandards.: General Fastener Standards BS OrderamdarvishvandNo ratings yet
- Dokumen - Tips Daewoo Service Manual Instrument Cluster Matiz-2023Document23 pagesDokumen - Tips Daewoo Service Manual Instrument Cluster Matiz-2023urexalg AlgériaNo ratings yet
- HE - Food and Beverages CGDocument14 pagesHE - Food and Beverages CGJohn Leonard88% (8)
- Water Hardness Case StudyDocument15 pagesWater Hardness Case StudyTaima GhNo ratings yet
- Pressure Vessel DesignDocument8 pagesPressure Vessel DesignHector Javier Cruz CampaNo ratings yet
- Gesell's Maturational TheoryDocument4 pagesGesell's Maturational TheorysanghuNo ratings yet
- Surgery Hazel Final1Document21 pagesSurgery Hazel Final1Sittie RamosNo ratings yet
- Ben T. Zinn Combustion LaboratoryDocument2 pagesBen T. Zinn Combustion LaboratoryLeslie WilliamsNo ratings yet
- Literature Review Sustainable DevelopmentDocument5 pagesLiterature Review Sustainable DevelopmentJade NelsonNo ratings yet
- CPC Sample Exam 1Document9 pagesCPC Sample Exam 1Renika_r80% (5)
- PROJECT IMS QEHS PLAN LEGAL REGISTERDocument4 pagesPROJECT IMS QEHS PLAN LEGAL REGISTERPriyanka JNo ratings yet
- Alkana-1Document61 pagesAlkana-1ayundhaNo ratings yet
- Diagnostic Test Science 5Document7 pagesDiagnostic Test Science 5Rex Russel SalemNo ratings yet
- SX SeriesDocument6 pagesSX SeriesmattuttezNo ratings yet
- Calcium chloride MSDSDocument5 pagesCalcium chloride MSDSDarshilNo ratings yet
- PAC and DELC indicate risk of heart attackDocument3 pagesPAC and DELC indicate risk of heart attackMatheus MoraisNo ratings yet
- Business Plan: Prepare by Hau Teen Yee FabriceDocument26 pagesBusiness Plan: Prepare by Hau Teen Yee FabricekarimanrlfNo ratings yet
- Lab Report 1 Biology PhotosynthesisDocument4 pagesLab Report 1 Biology PhotosynthesisSarthak PatelNo ratings yet
- Under The Oak Tree Part 2Document94 pagesUnder The Oak Tree Part 2suakasenaNo ratings yet