0% found this document useful (0 votes)
1K views6 pagesLinear Interpolation
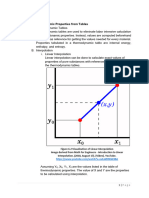
The document discusses linear interpolation techniques for determining thermodynamic properties of substances using tables of saturated vapor properties. It provides examples of using linear interpolation to find temperature, pressure, specific volume and quality for substances like R-12, ammonia, nitrogen, R-134a and water at given conditions by interpolating between nearby table values. Double linear interpolation is also demonstrated to find a property when two independent variables are given.
Uploaded by
stephen jamesCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
1K views6 pagesLinear Interpolation
The document discusses linear interpolation techniques for determining thermodynamic properties of substances using tables of saturated vapor properties. It provides examples of using linear interpolation to find temperature, pressure, specific volume and quality for substances like R-12, ammonia, nitrogen, R-134a and water at given conditions by interpolating between nearby table values. Double linear interpolation is also demonstrated to find a property when two independent variables are given.
Uploaded by
stephen jamesCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
- Problem 3.116: Describes how to find the pressure and temperature for saturated vapor R-12 using interpolation techniques.
- Problem 3.117: Covers estimation of ammonia properties using linear interpolation, including table filling exercise.
- Problem 3.118: Details the linear interpolation process to estimate temperature at a specified pressure for nitrogen.
- Problem 3.119: Explains the use of double linear interpolation to find pressure for superheated R-134a.
- Problem 3.120: Focuses on finding the specific volume of ammonia using interpolation from a given table.
- Problem 3.121: Involves calculating the pressure of water at specific conditions using given data tables.