Professional Documents
Culture Documents
COSTALD Correlation For Saturated Liquid Densities
Uploaded by
Prasad patgaonkarOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
COSTALD Correlation For Saturated Liquid Densities
Uploaded by
Prasad patgaonkarCopyright:
Available Formats
Correlation for Saturated Liquid Densities of
Hankinson and Thomson (COSTALD)
P. Maccone and J. Abusleme
Montefluos spa, via S. Pietro 50, 20021 Bollate (MI), Italy
Hankinson and Thomson (1979) developed a correlation for
saturated densities of liquids and their mixtures, which over- (7)
came mathematical discontinuity (Yen and Woods, 1966; Gunn
and Yamada, 1971) and pure compound limiting problems where Ak and B, are constants in Table 1.
(Spencer and Danner, 1972) in the equations used. In addition,
the COSTALD correlation leads to an average absolute percent Characteristic Volume, V*
error in pure compounds and mixtures liquid densities lower
In this work, we calculate the van der Waals volumes (V,)
than in the Yen-Woods and Spencer-Danner correlations.
with the group contributions proposed by Bondi (1968) for all
Moreover, COSTALD was later successfully extended to com-
pure compounds with V * determined with experimental liquid
pressed liquids and liquid mixtures (Thomson et al., 1982).
density data only (Hankinson and Thomson, 1979, Table 6).
These features make it possible to label this correlation as
A plot of V * vs. V,is shown in Figure 1. The clear correlation
highly predictive, accurate and reliable.
between the van der Waals and characteristic volumes observed
The aim of this work is to enhance the capability of pre-
in Figure 1 makes it worthwhile to propose an interpolation
diction of this correlation by introducing the group-contri-
equation as a function of V, to predict V’:
bution approach in the single adjustable parameter v*, called
the characteristic volume, required for each pure compound.
COSTALD Correlation
From Hankinson and Thomson (1979), we have the follow-
The C, parameters presented in Table 1 were determined using
ing pure compounds:
167 pure compounds giving an average absolute percent error
F to predict V * of 1.67%.
The characteristic volume can also be evaluated using the
following equation when no liquid density was available as
co)= 1+ c ,Ap(I -
4
0.25 < T,<0.95 suggested by Hankinson and Thomson:
TJk13 (2)
1
Val=
[: I/
x k B k T r (Tr-l.OOOO1) 0.25<T,<l.O (3)
Table 1. Parameters A,, Bkand C, of Eqs. 2, 3, and 8,
and for mixtures: respectively
cix j ~ i vftTcU)
~ j /V: (4)
k
0
A
-
B
- 0.29612 -
C
1 - 1.52816 0.38691 5.3850
2 1.43907 - 0.04273 - 5.1022E - 3
3 - 0.8 1446 - 0.04806 7.9524E - 5
4 0.19045 - - 9.93 16E - 8
5 - - 1.0088E - 10
6 - - - 1.1527E- 12
AIChE Journal March 1992 Vol. 38, No. 3 411
Table 3. Comparison of Eqs. 8-10 p Predict Characteristic
Volume, V
F=lOOIC[V * - V *(calc.)]/Y *Il/N
Groups F
N Eq. 8 Eq. 9 N Eq. 10
Paraffins 34 1.12 1.30 32 2.74
Olefins and 21 1.25 1.55 16 2.36
Diolefins
Cycloparaffins 8 3.04 4.01 8 1.51
Aromatics 8 1.75 0.90 7 0.76
Fluorocarbons 7 1.37 0.75 7 0.66
Sulfur compounds 5 0.49 4.50 4 1.92
50 100 150 300 All 83 1.38 1.73 74 2.10
Van der Waals Volume, Vw, (cc/mole)
Figure 1. Characteristicvolume V * determined with ex- Notation
perimentalliquid density and the van der Waals
A$&, = constants of Eqs. 2, 3 and 8, respectively
volume V,. a,b,c = parameters of Eq. 9, Hankinson and Thomson (1979,
Table 8)
N = number of pure compounds
where a, b, and c differ for each class of fluids (for example, R = gas constant
paraffins and aromatics). T = temperature, K
A third alternative to predict V * is to use the critical volume v = volume, cm’/mol
x = mole fraction
instead. The authors of COSTALD claimed that the discrep-
ancy between the characteristic volume and the critical volume
is very low. Thus, we also consider: Greek letter
wsRK = acentric factor from Soave equation of state
Superscript
Results * = characteristic
Table 2 presents for each class of fluid the average absolute
error percent given in Eq. 8 to predict V * , while Table 3 Subscripts
compares the performance of Eqs. 8,9 and 10. Unfortunately, c = critical property
Table 3 does not take into account as many systems as in Table
2 due to the lack of critical properties (Reid et al., 1977, 1987)
-
or the set a, b, and c required by Eq. 9. From Tables 2 and 0
COSTALD WITH EQUATION (8)
METHYL PENTYL ETHER
3, Eq. 8 holds the lowest error and the highest predictive A GI-n-BUTYL ETHER
capability. Equation 8 is not class-dependent and it uses only * GI-HEXYL ETHER
structural parameters. 0 PROPANOIC ACID
Figure 2 shows, for example, the saturated liquid density
predicted with COSTALD in Eq. 8 and the experimental data
of methyl-pentyl ether, di-butyl-ether and di-hexyl-ether (Ob-
ama et al., 1985) and propanoic acid (Bernardo-Gil et al.,
1990). For none of them, a, b, c and V * were available.
A
A
Table 2. Average Absolute 070 Error F of Eq. 8 to Predict --
Characteristic Volume, V *
F=1001C[V’- V*(calc.)]/Y *Il/N
0
~ ~ 0 0
Groups N F
Paraffins 40 1.28
Olefins and Diolefins 22 1.22
Cycloparaffins
Acetylenes
Aromatics
Fluorocarbons
Sulfur Compounds
10
4
13
7
57
2.69
5.67
1.30
1.37
0.74
.......
I I I I
Alcohols 4 3.36 50
Glycols 4 10.21 10 20 30 40 50
Others 6 4.71
Temperature, T, (‘C)
All 167 1.67
Figure 2. Saturated liquid volumes.
478 March 1992 Vol. 38, No. 3 AIChE Journal
i = component i Saturated Densities of Liquids and Their Mixtures,” AIChE J.,
m = mixture property 25(4), 653 (1979).
r = reduced property Obama, M., Y. Oodera, N. Kohama, T. Yanase, Y. Saito, and K.
s = saturated Kusano, “Densities, Molar Volumes, and Cubic Expansion Coef-
w = van der Waals ficients of 78 Aliphatic Ethers,” J. Chem. Eng. Data, 30, 1 (1985).
Reid, R. C., J. M. Prausnitz, and B. E. Poling, The Properties of
Gases and Liquids, 4th ed., McGraw-Hill, New York (1987).
Reid, R. C., J. M. Prausnitz, and T. K. Sherwood, The Properties
of Gases and Liquids, 3rd ed., McGraw-Hill, New York (1977).
Literature Cited Spencer, C. F., and R. P. Danner, “Improved Equation of Saturated
Bernardo-Gil, G., M. Esquivel, and A. Ribeiro, “Densities and Re- Liquid Density,” J. Chem. Eng. Data, 17, 236 (1972).
fractive Indices of Pure Organic Acids as a Function of Tempera- Thomson, G. H., K. R. Brobst, and R. W. Hankinson, “An Improved
ture,” J. Chem. Eng. Data, 35, 202 (1990). Correlation for Densities of Compressed Liquids and Liquids Mix-
Bondi, A., Physical Properties of Molecular Crystals, Liquids and tures,” AIChE J . , 28(4), 671 (1982).
Gases, Wiley, New York (1968). Yen, L. C., and S. S. Woods, “A Generalized Equation for Computer
Gunn, R. D., and T. Yamada, “A Corresponding States Correlation Calculation of Liquid Densities,” AIChE J., 12(1), 95 (1966).
of Saturated Liquid Volumes,” AIChE J., 17(6), 1341 (1971).
Hankinson, R. W., and G. H. Thomson, “A New Correlation for Manuscript received Sepi. 16, 1991.
~ ~~ ~
Errata
In the article titled “Reaction-Driven Convection in a Porous Medium” by Farr et al. (July
1991, p. 963), the plus sign in Eq. 55 should be a minus sign. All the results reported were calculated
with the correct equations.
In the article titled “Modeling of Reaction-Induced Flow Maldistributions in Packed Beds”
by Stroh and Balakotaiah (July 1991, p. 1035), tr in Eq. A13 should be E (complex conjugate). All
the results reported were calculated with the correct equations.
Equation 3 of the R&D note titled “Sorption with Oscillations in Solid Polymers” by Adib
and Neogi (January 1987, p. 164) should read:
2 2 (D65‘Yk-tI)*
I The figures in the note have been drawn using the correct equation.
AIChE Journal March 1992 Vol. 38, No. 3 479
You might also like
- International Thermodynamic Tables of the Fluid State: Propylene (Propene)From EverandInternational Thermodynamic Tables of the Fluid State: Propylene (Propene)No ratings yet
- Loufllgria: An Improved Correlation For Compressed Liquid Densities of Hydrocarbons. Part 2. MixturesDocument15 pagesLoufllgria: An Improved Correlation For Compressed Liquid Densities of Hydrocarbons. Part 2. MixturesPrasad patgaonkarNo ratings yet
- Costald 06-82Document6 pagesCostald 06-82boyd.george@bp.com100% (1)
- Mole Fraction Volume FractionDocument9 pagesMole Fraction Volume FractionameyckulkarniNo ratings yet
- GPA Standard 2174-93Document15 pagesGPA Standard 2174-93marcosNo ratings yet
- Costald 07-79Document11 pagesCostald 07-79boyd.george@bp.com100% (1)
- API Mpms 1121m 1984 Crude Oil CPL CalculationDocument4 pagesAPI Mpms 1121m 1984 Crude Oil CPL Calculationxjaf01No ratings yet
- Gpa 2174 - 93Document15 pagesGpa 2174 - 93jairo7662No ratings yet
- ASTM D5191 - Jtvo9242Document5 pagesASTM D5191 - Jtvo9242Nayth Andres GalazNo ratings yet
- Gpa STD 2177-2013Document18 pagesGpa STD 2177-2013Salvador Gomez50% (2)
- ZZ 1207573397 IsoFraction LNG Sampling SystemR2Document3 pagesZZ 1207573397 IsoFraction LNG Sampling SystemR2kaysb786133No ratings yet
- Cetano Norma D4737 10Document5 pagesCetano Norma D4737 10Jose Miguel GonzalezNo ratings yet
- LNG Sampler VaporizerDocument4 pagesLNG Sampler VaporizerDendeNo ratings yet
- Amspec Techtalk A Stands For API Gravity 4Document5 pagesAmspec Techtalk A Stands For API Gravity 4Sudea CadabaNo ratings yet
- API MPMS Version ControlDocument8 pagesAPI MPMS Version ControlMark Vincent EspinosaNo ratings yet
- Gas ship measurement guidelinesDocument40 pagesGas ship measurement guidelinesHenry MaedaNo ratings yet
- 39 14 The Safe Preparation of Gas MixturesDocument25 pages39 14 The Safe Preparation of Gas MixturesodonmarinesNo ratings yet
- ASTM D 1265 - 04 Standard Practice For Sampling Liquefied Petroleum (LP) Gases Manual Method PDFDocument5 pagesASTM D 1265 - 04 Standard Practice For Sampling Liquefied Petroleum (LP) Gases Manual Method PDFSaro HNo ratings yet
- Sampling Natural Gas ISO 10715Document34 pagesSampling Natural Gas ISO 10715Domingo PintoNo ratings yet
- Water Draw PresentationDocument52 pagesWater Draw PresentationManuel Liñeiro100% (1)
- Measurement ManualDocument83 pagesMeasurement ManualJavier Marcelo Sandoval BozoNo ratings yet
- Sampling Liquefied Petroleum (LP) Gases, Manual Method: Standard Practice ForDocument5 pagesSampling Liquefied Petroleum (LP) Gases, Manual Method: Standard Practice ForAhmedNo ratings yet
- Methane Number CalculationDocument7 pagesMethane Number CalculationMile ZoricNo ratings yet
- Software Applications: Flocalc Calculation DetailsDocument21 pagesSoftware Applications: Flocalc Calculation DetailsbabushleshaNo ratings yet
- What Is VEF (Vessel Experience Factor)Document2 pagesWhat Is VEF (Vessel Experience Factor)Giorgi KandelakiNo ratings yet
- Gpa 2186-05Document42 pagesGpa 2186-05Guiver Suarez V.No ratings yet
- Blending OilDocument7 pagesBlending OilAnonymous jqevOeP7No ratings yet
- GPA Table of Physical PropertiesDocument15 pagesGPA Table of Physical PropertiesMiissaEll Garciia100% (1)
- Ibc CHPT17 PDFDocument15 pagesIbc CHPT17 PDFKanwalahsan2017gmail.com Kanwalahsan2017gmail.comNo ratings yet
- ISO 14912 CorrectionsDocument3 pagesISO 14912 CorrectionsKazim RazaNo ratings yet
- D1560Document7 pagesD1560Aleksei AvilaNo ratings yet
- Gas Chromatography Analysis Speeds LPG Distillation: Analytical InstrumentationDocument2 pagesGas Chromatography Analysis Speeds LPG Distillation: Analytical InstrumentationCarolina OñateNo ratings yet
- Heating Value Estimation For Natural Gas ApplicationsDocument4 pagesHeating Value Estimation For Natural Gas Applicationspavanchem61No ratings yet
- FMC Technologies CalculationsDocument40 pagesFMC Technologies CalculationsKazuto KawakitaNo ratings yet
- Density and VCF CorrectionDocument12 pagesDensity and VCF Correctionfibber_gib100% (1)
- Gpa2261 19Document48 pagesGpa2261 19Porfirio Aguilera100% (1)
- Calculation of Vapor Pressure of A Gas MixtureDocument1 pageCalculation of Vapor Pressure of A Gas MixtureΓεωργια ΛεμενιτακηNo ratings yet
- Water in Crude Oil by Distillation: Standard Test Method ForDocument11 pagesWater in Crude Oil by Distillation: Standard Test Method ForMercedes de LarenaNo ratings yet
- Determination of C Through C Hydrocarbons in Gasolines by Gas ChromatographyDocument7 pagesDetermination of C Through C Hydrocarbons in Gasolines by Gas Chromatographyrimi7alNo ratings yet
- Density or Relative Density Test Method for Light HydrocarbonsDocument6 pagesDensity or Relative Density Test Method for Light HydrocarbonsGonzalo ChirinoNo ratings yet
- Accurately Calculate Nitrogen RequirementDocument6 pagesAccurately Calculate Nitrogen RequirementRachel BaileyNo ratings yet
- A BT CT: Viscosity of N-Haxane (C6H14)Document4 pagesA BT CT: Viscosity of N-Haxane (C6H14)Tri SulyonoNo ratings yet
- GAS QUALITY Methane Number Calculation 2012-04-04Document4 pagesGAS QUALITY Methane Number Calculation 2012-04-04Gabriel BerrioNo ratings yet
- ASTM D3700-14 MuestreoDocument11 pagesASTM D3700-14 MuestreomarcosNo ratings yet
- CO2 Capture Using Novel Biomass Derived Carbon DissertationDocument4 pagesCO2 Capture Using Novel Biomass Derived Carbon Dissertationali AbbasNo ratings yet
- Gpa 2174Document15 pagesGpa 2174Ceciliagorra100% (2)
- D1094 07 PDFDocument3 pagesD1094 07 PDFEmad AliNo ratings yet
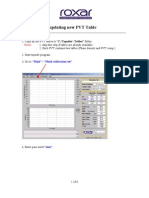
- Procedure For Down Loading PVT TableDocument6 pagesProcedure For Down Loading PVT TablejnizamarNo ratings yet
- Gas Properties: Molecular WeightDocument2 pagesGas Properties: Molecular WeightDamar WibisonoNo ratings yet
- ch8 PDFDocument144 pagesch8 PDFJuan ZamoraNo ratings yet
- Process Gas ChromatographRaphyDocument8 pagesProcess Gas ChromatographRaphyoviemeNo ratings yet
- Calculation of Z Factors For Natural Gases Using Equations of State P.M. Dranchuk J.H. Abou-KassemDocument4 pagesCalculation of Z Factors For Natural Gases Using Equations of State P.M. Dranchuk J.H. Abou-KassemAnonymous cCmpclQF6oNo ratings yet
- Guidelines For The Proper Application of Critical Velocity Calculations Sutton2010Document13 pagesGuidelines For The Proper Application of Critical Velocity Calculations Sutton2010nashat90No ratings yet
- NGA ISO 6974 ManualDocument89 pagesNGA ISO 6974 ManualhopkhtnNo ratings yet
- Compressibility For Non Ideal GasesDocument3 pagesCompressibility For Non Ideal Gasescymy100% (1)
- Iso 91 2017Document22 pagesIso 91 2017anitagissellatapiaNo ratings yet
- LNG Custody Transfer Handbook PDFDocument108 pagesLNG Custody Transfer Handbook PDFnavar001No ratings yet
- NSFMW 1992 Technical PapersDocument379 pagesNSFMW 1992 Technical Papersachmadh_2010No ratings yet
- Changing The Tool: Other Tools Can Be Downloaded From The GHG Protocol WebsiteDocument20 pagesChanging The Tool: Other Tools Can Be Downloaded From The GHG Protocol WebsiteDonn CorreaNo ratings yet
- CH 12 2 COLM Ballot 05 2015Document122 pagesCH 12 2 COLM Ballot 05 2015DougMooreNo ratings yet
- Heat Transfer in Agitated VesselsDocument33 pagesHeat Transfer in Agitated VesselsGanesh.Mahendra100% (1)
- Kinetics of Nitration of Aromatic Compounds With Mixed Acid Reinterpretation of Published DataDocument4 pagesKinetics of Nitration of Aromatic Compounds With Mixed Acid Reinterpretation of Published DataPrasad patgaonkarNo ratings yet
- Nitration Reactions in Pharma IntermediatesDocument7 pagesNitration Reactions in Pharma IntermediatesPrasad patgaonkarNo ratings yet
- Impeller Design For Mixing of SuspensionsDocument16 pagesImpeller Design For Mixing of SuspensionsFaycel OuerdienNo ratings yet
- Xcos BeginnersDocument15 pagesXcos BeginnersJorge Eduardo SepúlvedaNo ratings yet
- Sizing of Pressure Relief ValveDocument9 pagesSizing of Pressure Relief Valvekenoly123100% (3)
- McCabe SciLab CodesDocument176 pagesMcCabe SciLab Codesre1se2arch4No ratings yet
- Scilab BeginnersDocument33 pagesScilab BeginnersCarlos Soza RossNo ratings yet
- Chemical Engineering Thermodynamics-2Document32 pagesChemical Engineering Thermodynamics-2Saif Ullah KamranNo ratings yet
- SRK EOS NEW PARAMETER MIXING RULES-SoaveDocument14 pagesSRK EOS NEW PARAMETER MIXING RULES-SoavePrasad patgaonkarNo ratings yet
- Physical PropertiesDocument121 pagesPhysical PropertiesSampathkumar AttuluriNo ratings yet
- Physical PropertiesDocument121 pagesPhysical PropertiesSampathkumar AttuluriNo ratings yet
- Calculating BIP in PR EoS PDFDocument9 pagesCalculating BIP in PR EoS PDFPrasad patgaonkarNo ratings yet
- Calculating BIP in PR EoS PDFDocument9 pagesCalculating BIP in PR EoS PDFPrasad patgaonkarNo ratings yet
- Calculating BIP in PR EoS PDFDocument9 pagesCalculating BIP in PR EoS PDFPrasad patgaonkarNo ratings yet
- A Class of Methods For Solving Nonlinear Simultaneous EquationsDocument18 pagesA Class of Methods For Solving Nonlinear Simultaneous EquationsPrasad patgaonkarNo ratings yet
- A Class of Methods For Solving Nonlinear Simultaneous EquationsDocument18 pagesA Class of Methods For Solving Nonlinear Simultaneous EquationsPrasad patgaonkarNo ratings yet
- Calculating BIP in PR EoS PDFDocument9 pagesCalculating BIP in PR EoS PDFPrasad patgaonkarNo ratings yet
- Lesson Plan For Demonstration (COT) : School Grade Level Teacher Learning Area Teaching Dates and Time QuarterDocument6 pagesLesson Plan For Demonstration (COT) : School Grade Level Teacher Learning Area Teaching Dates and Time Quartermaritel dawaNo ratings yet
- The Management of Productivity and Technology in Manufacturing PDFDocument333 pagesThe Management of Productivity and Technology in Manufacturing PDFmythee100% (2)
- Unit 2: Marketing Processes and Planning: Assignment BriefDocument4 pagesUnit 2: Marketing Processes and Planning: Assignment BriefGharis SoomroNo ratings yet
- Open University of Tanzania Term PaperDocument7 pagesOpen University of Tanzania Term Paperafmzfmeeavndqe100% (1)
- DDX3035 - Audio File - KenwoodDocument5 pagesDDX3035 - Audio File - KenwoodRistho LutherNo ratings yet
- Legal Documents Evidence in Safety Related Proceedings 1663086621Document9 pagesLegal Documents Evidence in Safety Related Proceedings 1663086621richardNo ratings yet
- SEO-Optimized Title for Document on English Exam QuestionsDocument4 pagesSEO-Optimized Title for Document on English Exam Questionsminh buiNo ratings yet
- Ho'oponopono ! - The Power of ForgivenessDocument6 pagesHo'oponopono ! - The Power of ForgivenessParainNo ratings yet
- How English Works - A Grammar Handbook With Readings - Answer Key PDFDocument38 pagesHow English Works - A Grammar Handbook With Readings - Answer Key PDFAlessio Bocco100% (1)
- I Am Sharing 'ASSIGNMENT MAT099 - E08G5 - WRTTEN REPORT' With YouDocument23 pagesI Am Sharing 'ASSIGNMENT MAT099 - E08G5 - WRTTEN REPORT' With YouaqilNo ratings yet
- Text 1 Is For No 1 - 4 The Rabbit Revenge: I. Pilihlah Salah Satu Jawaban Yang Benar!Document4 pagesText 1 Is For No 1 - 4 The Rabbit Revenge: I. Pilihlah Salah Satu Jawaban Yang Benar!Diandra MomentNo ratings yet
- EL FILI CHAPTER 13Document9 pagesEL FILI CHAPTER 13Eduardo Sismundo JrNo ratings yet
- Header LpuDocument3 pagesHeader LpuL.a.ZumárragaNo ratings yet
- Accenture Sustainable ProcurementDocument2 pagesAccenture Sustainable ProcurementAnupriyaSaxenaNo ratings yet
- Quotation SS20230308 100KVAR APFC PANEL VIDHYA WIRESDocument4 pagesQuotation SS20230308 100KVAR APFC PANEL VIDHYA WIRESsunil halvadiyaNo ratings yet
- Biotensegrity and Myofascial Chains A Global Approach To An Integrated Kinetic ChainDocument8 pagesBiotensegrity and Myofascial Chains A Global Approach To An Integrated Kinetic ChainMohamed ElMeligieNo ratings yet
- 01 A Beginner's Guide To TajikiDocument384 pages01 A Beginner's Guide To Tajikitaryuman100% (1)
- 21eb7 0 PDFDocument1 page21eb7 0 PDFdediranduNo ratings yet
- AGN PresentationDocument119 pagesAGN PresentationNikki GarlejoNo ratings yet
- Medical Services Recruitment Board (MRB)Document20 pagesMedical Services Recruitment Board (MRB)durai PandiNo ratings yet
- Small BusinessDocument22 pagesSmall BusinessAngelie Dela CruzNo ratings yet
- Earned Value Analysis 8 StepsDocument8 pagesEarned Value Analysis 8 StepsHira RazzaqNo ratings yet
- LPI PH PDFDocument4 pagesLPI PH PDFHumberto Tapias CutivaNo ratings yet
- Central Bank Digital Currency:: The Future of Payments For CorporatesDocument29 pagesCentral Bank Digital Currency:: The Future of Payments For CorporatesknwongabNo ratings yet
- International Standard Iso/Iec Software Cycle Processes: Standards SectionDocument16 pagesInternational Standard Iso/Iec Software Cycle Processes: Standards SectionDavid SalgadoNo ratings yet
- Google Wakeword Detection 1 PDFDocument5 pagesGoogle Wakeword Detection 1 PDFÖzgür Bora GevrekNo ratings yet
- DTC Codes Mercedes CPC4 EnglishDocument24 pagesDTC Codes Mercedes CPC4 Englishjonny david martinez perez100% (1)
- Leading Causes of MortalityDocument12 pagesLeading Causes of MortalityJayricDepalobosNo ratings yet
- Holzma PR Hob and Ex Linger Enu 18533Document7 pagesHolzma PR Hob and Ex Linger Enu 18533esfandiar232477No ratings yet
- Banking and Fintech in 2022Document45 pagesBanking and Fintech in 2022Shahbaz talpurNo ratings yet