Professional Documents
Culture Documents
Chapter 10. Practice Problems Part I. True or False
Uploaded by
Alwyn Dave Ambatali0 ratings0% found this document useful (0 votes)
10 views1 pageThis document contains practice problems about acid-base chemistry concepts. It has three parts: 1) True/False questions about Arrhenius, Bronsted-Lowry, and Lewis acid-base definitions; 2) Questions about acid strength and identifying strong vs. weak acids/bases; 3) Problems calculating pH, pOH, [H+], and [OH-] given concentration information.
Original Description:
Original Title
ACIDS AND BASES
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains practice problems about acid-base chemistry concepts. It has three parts: 1) True/False questions about Arrhenius, Bronsted-Lowry, and Lewis acid-base definitions; 2) Questions about acid strength and identifying strong vs. weak acids/bases; 3) Problems calculating pH, pOH, [H+], and [OH-] given concentration information.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views1 pageChapter 10. Practice Problems Part I. True or False
Uploaded by
Alwyn Dave AmbataliThis document contains practice problems about acid-base chemistry concepts. It has three parts: 1) True/False questions about Arrhenius, Bronsted-Lowry, and Lewis acid-base definitions; 2) Questions about acid strength and identifying strong vs. weak acids/bases; 3) Problems calculating pH, pOH, [H+], and [OH-] given concentration information.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
CHAPTER 10.
PRACTICE PROBLEMS
Part I. True or False.
1. The Arrhenius definition defines acid as a substance that adds [ H+ ] in aqueous
solution while base as a substance that adds [OH-] in aqueous solution.
2. Using the Arrhenius definition, we could prove that NH3 is a base.
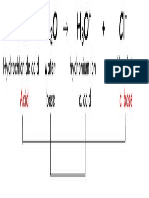
3. In the Bronsted- Lowry definition, an acid accepts H+ and a base donates H+.
4. The reaction of NH3 + H2O à NH4+ + OH-, NH3 is the Bronsted- Lowry acid and H2O is
the base.
5. The reaction of NH3 + H2O à NH4+ + OH-, NH4+ is conjugate acid acid and OH- is the
conjugate base.
6. The Lewis Definition explains acid as an electron pair-donor while the base as an
electron- pair acceptor.
7. In the reaction NH3 + HCl à NH4Cl , NH3 is a lewis base while HCl is a lewis acid.
8. In the reaction NH3 + HCl à NH4Cl , NH4Cl is an adduct.
9. The pH of a strong base is closer to 14 while the pH of a strong acid is closer to 0.
10. The pOH of a substance is a measure of the basicity of a substance.
Part II. Answer the following.
1. Arrange the following in increasing acid strength:
a. HF, HCl, HI, HBr
b. HClO, HClO3, HClO2, HClO4
c. H3PO4 , H2SO4, H3PO3, H2SO3
2. Which of the following is not a strong acid?
a. HF, HCl, HNO3, H2SO4
b. H3PO4, HCN, HClO4
3. Which of the following is not a strong base?
a. NH3, NaOH, KOH
b. Sr(OH)2, (NH2)2CO, Ba(OH)2
c. Ca(OH)2, LiOH, CH3NH
Part III. Solve the following.
1. The concentration of H+ ions in a bottle of wine was 1.0x10-6 M right after the cork was
removed. Only half of the wine was consumed.
a. Calculate the pH, pOH and [OH-]
b. The other half, after standing open to the air for a month, was found to have a [H+] of
1.0x10-3 M. Calculate the pH, pOH and [OH-]
2. The pH of rainwater collected was 5.00. Calculate the following: pOH, [H+], [OH-]
You might also like
- Acids & Bases Lecture NotesDocument51 pagesAcids & Bases Lecture NotesTahir Hussain100% (1)
- 3 Acids & BasesDocument24 pages3 Acids & BasesAbdul Quddus100% (1)
- Acid BaseDocument70 pagesAcid BasevitrekNo ratings yet
- General Chemistry Notes For SHS PDFDocument20 pagesGeneral Chemistry Notes For SHS PDFAlwyn Dave Ambatali100% (4)
- Recent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumFrom EverandRecent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumW. D. OllisNo ratings yet
- Test2 Ch17a Acid-Base Practice Problems PDFDocument12 pagesTest2 Ch17a Acid-Base Practice Problems PDFRaphael CastilloNo ratings yet
- Module 4 - Acids and BasesDocument7 pagesModule 4 - Acids and BasesRuth Aquino100% (1)
- Acids and BasesDocument33 pagesAcids and BasesFrancene Badana YepesNo ratings yet
- Study CheckDocument1 pageStudy CheckJenelyn Ponce AguiloNo ratings yet
- Proton - 2 - Acids and BasesDocument36 pagesProton - 2 - Acids and BasesFrancene Badana YepesNo ratings yet
- Acid-Base Equilibria: Chemistry Grade 12 Monthly Note 2nd UNIT-2Document23 pagesAcid-Base Equilibria: Chemistry Grade 12 Monthly Note 2nd UNIT-2Tebarek SitotawNo ratings yet
- ACID BASE THEORY by FS ShahDocument24 pagesACID BASE THEORY by FS Shahfarooq shah shabbirNo ratings yet
- Acid-Base Practice ProblemsDocument12 pagesAcid-Base Practice ProblemsDAKSH CHETAN HATHINo ratings yet
- Chemistry Chapter 10Document31 pagesChemistry Chapter 10Misbah JilaniNo ratings yet
- Acids, Bases and Non-Aqueous Solvents PDFDocument27 pagesAcids, Bases and Non-Aqueous Solvents PDFak fuad0% (1)
- Inorganic Cha 4Document21 pagesInorganic Cha 4Adugnaw BiksNo ratings yet
- Acid-Base Chemistry: Manasi MantriDocument16 pagesAcid-Base Chemistry: Manasi MantriSonam ChhedaNo ratings yet
- Ionic Equilibria Acids and Bases NotesDocument21 pagesIonic Equilibria Acids and Bases Notesseanapollomarco.cantosNo ratings yet
- 13 Ionic Equilibria Notes PDFDocument37 pages13 Ionic Equilibria Notes PDFUchiha YogesNo ratings yet
- Ionic EquilibriumDocument23 pagesIonic EquilibriumjamzeethsNo ratings yet
- Titulaciones Acuosas y No AcuosasDocument27 pagesTitulaciones Acuosas y No AcuosasMayerli LeónNo ratings yet
- Module in Acid-BasesDocument8 pagesModule in Acid-BasesPenPen MalayNo ratings yet
- 8 2 Bronsted-Lowry Acids and BasesDocument27 pages8 2 Bronsted-Lowry Acids and BasesLyka BugarinNo ratings yet
- 8 2 Bronsted-Lowry Acids and BasesDocument13 pages8 2 Bronsted-Lowry Acids and Basesribots2002No ratings yet
- Conjugate Acid Base Pairs: Name - Chem Worksheet 19-2Document1 pageConjugate Acid Base Pairs: Name - Chem Worksheet 19-2Bindu Mohanan PillaiNo ratings yet
- UNIT 2.docx Grade 12 Chemistry Note and WSDocument27 pagesUNIT 2.docx Grade 12 Chemistry Note and WSmesfin yonasNo ratings yet
- Cursul 7Document32 pagesCursul 7Bogdan Cel MicNo ratings yet
- 5th Meeting - Acid N Base Titration, Buffer, Hydrolisis Soolubilty ProductsDocument54 pages5th Meeting - Acid N Base Titration, Buffer, Hydrolisis Soolubilty ProductsCantika WulandariNo ratings yet
- Acid Base ConceptDocument20 pagesAcid Base Conceptyadavamlesh045No ratings yet
- 7.1 Acids and Bases 22-23 PDFDocument143 pages7.1 Acids and Bases 22-23 PDFTomatoNo ratings yet
- Acid and BasesDocument4 pagesAcid and BasesAina HaravataNo ratings yet
- Z0217002012017408710 - Acid and Bases - Revision 1Document44 pagesZ0217002012017408710 - Acid and Bases - Revision 1joenni hansNo ratings yet
- Acids and Bases Review Hon-18Document2 pagesAcids and Bases Review Hon-18api-368121935No ratings yet
- GenChem2 Q4 MELC 7-9 Week-5Document7 pagesGenChem2 Q4 MELC 7-9 Week-5BSED FIL 1- Ashley Romarie A. LactaotaoNo ratings yet
- Gen Chem 2-Q4-Week 3-V1Document9 pagesGen Chem 2-Q4-Week 3-V1Ivyy Joyce BuanNo ratings yet
- 7.0 Ionic EquilibriaDocument124 pages7.0 Ionic EquilibriaTasya KassimNo ratings yet
- Acid Base Intro Powerpoint 2020Document35 pagesAcid Base Intro Powerpoint 2020JulesNo ratings yet
- 6-Acids and BasesDocument46 pages6-Acids and Basesnirvanjain212007No ratings yet
- Video NotesDocument45 pagesVideo Notesjim tannerNo ratings yet
- Conjugate Acid and Base PairsDocument1 pageConjugate Acid and Base Pairsfelisita wisangNo ratings yet
- Unit 3 Ionic EquibliriumDocument63 pagesUnit 3 Ionic EquibliriumFiixaa B OlqabaaNo ratings yet
- Chapter 2 Acid - Base - EngDocument98 pagesChapter 2 Acid - Base - Englong.vuongbz188No ratings yet
- GC2 Q4 L4stemDocument45 pagesGC2 Q4 L4stemeli candazaNo ratings yet
- Acid-Base Def & StrengthsDocument12 pagesAcid-Base Def & StrengthsCheu Hann JongNo ratings yet
- Country's Best Online Test PlatformDocument63 pagesCountry's Best Online Test PlatformSubhrasankar RaychaudhuryNo ratings yet
- Chapter 7Document259 pagesChapter 7Hafizszul Feyzul100% (1)
- Teori Asam Basa (B.inggris)Document31 pagesTeori Asam Basa (B.inggris)Lukman Al AminNo ratings yet
- CHM271 - Chapter 3 - Ionic EquilibriumDocument49 pagesCHM271 - Chapter 3 - Ionic Equilibriumnur artikaNo ratings yet
- Ionic EquilibriumDocument46 pagesIonic EquilibriumPadmalaya paloNo ratings yet
- Lesson 1 NotesDocument6 pagesLesson 1 Notesnandini.e1809No ratings yet
- Bronsted LowryDocument71 pagesBronsted LowryShaina NovicioNo ratings yet
- Chapter 3Document124 pagesChapter 3Fariz SharudinNo ratings yet
- Acid Base Notes (Use For Chem 123)Document16 pagesAcid Base Notes (Use For Chem 123)Nate JamesNo ratings yet
- Acid Ionic EqulbrmDocument21 pagesAcid Ionic EqulbrmsheenajerryNo ratings yet
- Unit 11 Packet PDFDocument25 pagesUnit 11 Packet PDFMaxim HristozovNo ratings yet
- General Organic and Biological Chemistry 6Th Edition Stoker Solutions Manual Full Chapter PDFDocument37 pagesGeneral Organic and Biological Chemistry 6Th Edition Stoker Solutions Manual Full Chapter PDFyhenryhnorc5100% (7)
- Chemistry NotesDocument7 pagesChemistry NotesMARYA KHALIDNo ratings yet
- Bronsted-Lowry Acids and BasesDocument2 pagesBronsted-Lowry Acids and Basessikab31632No ratings yet
- Chm096 Chapter 4 Acids and BasesDocument257 pagesChm096 Chapter 4 Acids and Basessalihah95No ratings yet
- Ionic Equilibria (Part 1)Document33 pagesIonic Equilibria (Part 1)Timothy HandokoNo ratings yet
- Genchem Notes For Freshmen PDFDocument7 pagesGenchem Notes For Freshmen PDFAlwyn Dave AmbataliNo ratings yet
- Simple Calculations and SigfigsDocument2 pagesSimple Calculations and SigfigsAlwyn Dave AmbataliNo ratings yet
- LBYPH03Document2 pagesLBYPH03Alwyn Dave AmbataliNo ratings yet
- Chapter 6. Practice Problems: 1 1 2 2 1 1 1 1 2 2 2 2 1 1 2 2 1 1 2 2 1 1 2 2 Total 1 2 1 1 TDocument3 pagesChapter 6. Practice Problems: 1 1 2 2 1 1 1 1 2 2 2 2 1 1 2 2 1 1 2 2 1 1 2 2 Total 1 2 1 1 TAlwyn Dave AmbataliNo ratings yet
- Kinetics Notes PDFDocument3 pagesKinetics Notes PDFAlwyn Dave AmbataliNo ratings yet
- Bonds and Type of ReactionsDocument7 pagesBonds and Type of ReactionsAlwyn Dave AmbataliNo ratings yet
- Assignment-Chapters 1 To 3 Part 1. Complete The Table: Element Electron Configuration Orbital Diagram Quantum NumbersDocument4 pagesAssignment-Chapters 1 To 3 Part 1. Complete The Table: Element Electron Configuration Orbital Diagram Quantum NumbersAlwyn Dave AmbataliNo ratings yet
- Chapter 9. Practice Problems Part I. Refer To The Following Chemical Equation: 2A + 3B 4C + 5DDocument4 pagesChapter 9. Practice Problems Part I. Refer To The Following Chemical Equation: 2A + 3B 4C + 5DAlwyn Dave AmbataliNo ratings yet
- Chapter 12. Practice Problems Part I. Do As IndicatedDocument2 pagesChapter 12. Practice Problems Part I. Do As IndicatedAlwyn Dave AmbataliNo ratings yet
- Phosphorus and IodineDocument3 pagesPhosphorus and IodineAlwyn Dave AmbataliNo ratings yet
- Document 9 PDFDocument2 pagesDocument 9 PDFAlwyn Dave AmbataliNo ratings yet