Professional Documents
Culture Documents
Conjugate Acid Base Pairs: Name - Chem Worksheet 19-2
Uploaded by
Bindu Mohanan PillaiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Conjugate Acid Base Pairs: Name - Chem Worksheet 19-2
Uploaded by
Bindu Mohanan PillaiCopyright:
Available Formats
Conjugate Acid Base Pairs Name ________________
Chem Worksheet 19-2
An acid is defined as a proton (H+) donor while a base is a proton
Acids donate protons
acceptor. The substance that is produced after an acid has donated its
Bases accept protons
proton is called the conjugate base while the substance formed when a
base accepts a proton is called the conjugate acid. The conjugate acid
A proton is a hydrogen ion
can donate a proton to the conjugate base, to reform the original
reactants in the reverse reaction.
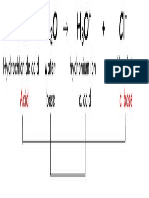
HF + H2O ! H3O+ + F–
acid base c. acid c. base
In the reaction above HF is the acid and H2O is the base. The HF has given a proton to the H2O,
forming H3O+ and F–. Since the product H3O+ can donate a proton back to F– it is labeled the conjugate
acid, while the F– is the conjugate base.
Example
Write an equation that shows NH3 reacting with HCl. Label the acid, base, and conjugate acid and conjugate base.
H+
- Write reactants and transfer a proton from the acid to the base: NH3 + HCl ! NH4+ + Cl–
base acid c. acid c. base
Rewrite each equation. Identify the acid, the base, the conjugate acid, and the conjugate
base in each of the equations.
1. HCl + NH3 ! NH4+ + Cl– 5. HCO3– + OH– ! H2O + CO32–
2. OH– + HCN ! H2O + CN– 6. NH4+ + H2O ! NH3 + H3O+
3. PO43– + HNO3 ! NO3– + HPO42– 7. C2O42– + HC2H3O2 ! HC2O4– + C2H3O2–
4. HCO3– + HCl ! H2CO3 + Cl– 8. HPO42– + H2O ! OH– + H2PO4–
Fill in the following table.
Acid Base Conjugate Acid Conjugate Base Equation
9 HNO2 H2O HNO2 + H2O ! NO2– + H3O+
10 H2O F– HF OH–
11 NH3 + HCN ! NH4+ + CN–
12 H2O ClO3–
13 HSO4– PO43–
14 S2– + H2O ! OH– + HS–
15 HCO2H OH-
16. Write an equation that shows the reaction of ammonia, NH3 with hydrobromic acid, HBr. Label the acid,
the base, the conjugate acid, and the conjugate base.
17. Write an equation that shows the reaction of phosphate ion, PO43–, reacting with hydronium ion, H3O+.
Label the acid, the base, the conjugate acid, and the conjugate base.
18. Write an equation that shows the reaction of hydrogen sulfide, HS– with hydroxide ion, OH–. Label the
acid, the base, the conjugate acid, and the conjugate base.
© John Erickson, 2005 WS19-2ConjugatePairs
You might also like
- Construction and Application of Heat SensorDocument42 pagesConstruction and Application of Heat Sensorokereke ebuka87% (23)
- Conjugate Acid Base Pairs: Name - Chem Worksheet 19-2Document2 pagesConjugate Acid Base Pairs: Name - Chem Worksheet 19-2Taylor Delancey100% (2)
- 4.8 Introduction To Acid-Base Reactions StudentDocument3 pages4.8 Introduction To Acid-Base Reactions StudentSyed RazaNo ratings yet
- Conjugate Acid and Base PairsDocument1 pageConjugate Acid and Base Pairsfelisita wisangNo ratings yet
- Acids and Bases Song Explains Conjugate PairsDocument27 pagesAcids and Bases Song Explains Conjugate PairsLyka BugarinNo ratings yet
- Chapter 3 Acid - BaseDocument98 pagesChapter 3 Acid - BasePHƯƠNG ĐẶNG YẾNNo ratings yet
- Acid Ionic EqulbrmDocument21 pagesAcid Ionic EqulbrmsheenajerryNo ratings yet
- Acid-Base Practice ProblemsDocument12 pagesAcid-Base Practice ProblemsDAKSH CHETAN HATHINo ratings yet
- Acids and Bases Chapter SummaryDocument13 pagesAcids and Bases Chapter Summaryribots2002No ratings yet
- Chapter 3 Acid - BaseDocument98 pagesChapter 3 Acid - BaseKhoa Nguyen Viet DangNo ratings yet
- Titulaciones Acuosas y No AcuosasDocument27 pagesTitulaciones Acuosas y No AcuosasMayerli LeónNo ratings yet
- Acid Base ConceptDocument20 pagesAcid Base Conceptyadavamlesh045No ratings yet
- Study CheckDocument1 pageStudy CheckJenelyn Ponce AguiloNo ratings yet
- Chapter 10. Practice Problems Part I. True or FalseDocument1 pageChapter 10. Practice Problems Part I. True or FalseAlwyn Dave AmbataliNo ratings yet
- Acid/Base Equilibria - Chapter 16Document19 pagesAcid/Base Equilibria - Chapter 16aniedorfNo ratings yet
- Inorganic Cha 4Document21 pagesInorganic Cha 4Adugnaw BiksNo ratings yet
- Chapter 3 Acid - BaseDocument96 pagesChapter 3 Acid - BaseAnh NhamNo ratings yet
- Unit 3 Ionic EquibliriumDocument63 pagesUnit 3 Ionic EquibliriumFiixaa B OlqabaaNo ratings yet
- Acid BaseDocument70 pagesAcid BasevitrekNo ratings yet
- Topic 12 NotesDocument31 pagesTopic 12 NotesHassan 2No ratings yet
- Ionic Equilibria and pH CalculationsDocument124 pagesIonic Equilibria and pH CalculationsTasya KassimNo ratings yet
- Test2 Ch17a Acid-Base Practice Problems PDFDocument12 pagesTest2 Ch17a Acid-Base Practice Problems PDFRaphael CastilloNo ratings yet
- PPP1B 8.1 B-L Acids BasesDocument20 pagesPPP1B 8.1 B-L Acids BasesRichard LindemannNo ratings yet
- Country's Best Online Test PlatformDocument63 pagesCountry's Best Online Test PlatformSubhrasankar RaychaudhuryNo ratings yet
- NH 3 H 2 o Oh NH 4 Acid Base Concepts Chapter 15 H Conjugate Acid Base Pairs H Base AcidDocument12 pagesNH 3 H 2 o Oh NH 4 Acid Base Concepts Chapter 15 H Conjugate Acid Base Pairs H Base AcidKhang TrầnNo ratings yet
- Topic 12 NotesDocument31 pagesTopic 12 NotesHK Nova ChiuNo ratings yet
- Chapter 2 Acid - Base - EngDocument98 pagesChapter 2 Acid - Base - Englong.vuongbz188No ratings yet
- Chapter 3 Acid - BaseDocument98 pagesChapter 3 Acid - BaseNhan PhướcNo ratings yet
- PH and Acid-Base Reactions PDFDocument54 pagesPH and Acid-Base Reactions PDFShifa RizwanNo ratings yet
- PH and Acid-Base Reactions PDFDocument54 pagesPH and Acid-Base Reactions PDFZenonissya GalwanNo ratings yet
- Ionic Equilibria: pH CalculationsDocument37 pagesIonic Equilibria: pH CalculationsUchiha YogesNo ratings yet
- Modul 2 (Asam Basa)Document40 pagesModul 2 (Asam Basa)Seo HerinNo ratings yet
- 5th Meeting_Acid n Base Titration, Buffer, Hydrolisis Soolubilty ProductsDocument54 pages5th Meeting_Acid n Base Titration, Buffer, Hydrolisis Soolubilty ProductsCantika WulandariNo ratings yet
- Acids and Bases Zapper PresDocument40 pagesAcids and Bases Zapper PresAgung PratamaNo ratings yet
- Chapter 3 - Concept of Acid-Base NeutralisationDocument58 pagesChapter 3 - Concept of Acid-Base NeutralisationIkmal FikriNo ratings yet
- Module in Acid-BasesDocument8 pagesModule in Acid-BasesPenPen MalayNo ratings yet
- Chapter 7 Acid-Base ReactionDocument111 pagesChapter 7 Acid-Base ReactionUMMU MARDHIAH ABDUL HALIMNo ratings yet
- Acid Base Equilibria - NotesDocument6 pagesAcid Base Equilibria - NotesNur Afiqah Mohd ZakiNo ratings yet
- Activity 5 Acid Base Equilibria ADRIANNENENG1 ANSWERSDocument8 pagesActivity 5 Acid Base Equilibria ADRIANNENENG1 ANSWERSadrian nenengNo ratings yet
- Acid Base Intro Powerpoint 2020Document35 pagesAcid Base Intro Powerpoint 2020JulesNo ratings yet
- Acids and Bases Review Hon-18Document2 pagesAcids and Bases Review Hon-18api-368121935No ratings yet
- Acids BasesDocument30 pagesAcids BasesHaniel GalzoteNo ratings yet
- 1-Neutralization Theory2Document24 pages1-Neutralization Theory2watersoul.nNo ratings yet
- Inorganic Chemistry: Acids, Bases and Non-Aqueous SolventsDocument27 pagesInorganic Chemistry: Acids, Bases and Non-Aqueous Solventsak fuad0% (1)
- Chapter 3Document124 pagesChapter 3Fariz SharudinNo ratings yet
- 9.1.conjugate Acid-Base PairsDocument11 pages9.1.conjugate Acid-Base PairsFelicia GunawanNo ratings yet
- Ionic EquilibriumDocument60 pagesIonic EquilibriumVermavinay3940 vinay394080% (5)
- NS1Lec - Module 5 - NacionalesDocument5 pagesNS1Lec - Module 5 - NacionalesWindere Marie NacionalesNo ratings yet
- Bronsted Lowry+WorksheetDocument4 pagesBronsted Lowry+WorksheetHo Hsiao JiunNo ratings yet
- General Organic and Biological Chemistry 6Th Edition Stoker Solutions Manual Full Chapter PDFDocument37 pagesGeneral Organic and Biological Chemistry 6Th Edition Stoker Solutions Manual Full Chapter PDFyhenryhnorc5100% (7)
- Chapter 7Document259 pagesChapter 7Hafizszul Feyzul100% (1)
- Acid-Base TitrationDocument150 pagesAcid-Base TitrationKukkiboNo ratings yet
- Ionic EquilibriumDocument46 pagesIonic EquilibriumPadmalaya paloNo ratings yet
- Chemistry Chapter 2Document160 pagesChemistry Chapter 2Deepak vishalNo ratings yet
- Summary Topic 8 Acids and BasesDocument9 pagesSummary Topic 8 Acids and BasesNubar MammadovaNo ratings yet
- CHEM2-LEC4 Acid Base EquilibriaDocument40 pagesCHEM2-LEC4 Acid Base EquilibriaAlphonse SambranoNo ratings yet
- bcm.06 Acids and BasesDocument29 pagesbcm.06 Acids and BaseslauderNo ratings yet
- Acids & Bases PropertiesDocument3 pagesAcids & Bases PropertiesVon Joby RomeroNo ratings yet
- The Chemistry of Acids and BasesDocument68 pagesThe Chemistry of Acids and BasesHelpful Hand100% (1)
- Tobias, Mark Denzel B. Bse 1B Science: Worksheet - Bronsted-Lowry Acids and Bases Name: Year and Section: DateDocument2 pagesTobias, Mark Denzel B. Bse 1B Science: Worksheet - Bronsted-Lowry Acids and Bases Name: Year and Section: DateMaden betoNo ratings yet
- TERM2 - EXAMINATION - 2020 - 21 - Grade X - PHYSICS (E22MNV) (With Markscheme)Document32 pagesTERM2 - EXAMINATION - 2020 - 21 - Grade X - PHYSICS (E22MNV) (With Markscheme)Bindu Mohanan PillaiNo ratings yet
- Acids & Bases - SLDocument7 pagesAcids & Bases - SLBindu Mohanan PillaiNo ratings yet
- Paper-1 - Higher Level - Multiple Choice Question Total Marks15 Marks Date-28 July 2022 Time:15 MinutesDocument9 pagesPaper-1 - Higher Level - Multiple Choice Question Total Marks15 Marks Date-28 July 2022 Time:15 MinutesBindu Mohanan PillaiNo ratings yet
- Development ReportDocument28 pagesDevelopment ReportBindu Mohanan PillaiNo ratings yet
- 0002 - 709 Gas Testing Using Portable Gas MonitorsDocument12 pages0002 - 709 Gas Testing Using Portable Gas MonitorsSrinivas Maddenapelli100% (2)
- Excursions in Statistical Dynamics: Gavin E. CrooksDocument117 pagesExcursions in Statistical Dynamics: Gavin E. CrookspzvpzvNo ratings yet
- Thermodynamics Lecture SummaryDocument26 pagesThermodynamics Lecture SummaryHan VendiolaNo ratings yet
- Lec3 PLCDocument36 pagesLec3 PLCmayarm802No ratings yet
- Lecture - Electron DiffractionDocument12 pagesLecture - Electron DiffractionOlivia WahyudiNo ratings yet
- Unit 2 ElectrostaticDocument2 pagesUnit 2 ElectrostaticJainav SanghviNo ratings yet
- 9 Science TP 12 1Document5 pages9 Science TP 12 1SaurabhNo ratings yet
- Plate Tectonics: Eden M Napo Science TeacherDocument27 pagesPlate Tectonics: Eden M Napo Science TeacherMary Rose FajardoNo ratings yet
- Wellbore CalculationsDocument34 pagesWellbore Calculationsbaskr82100% (1)
- Answer Sa StemDocument7 pagesAnswer Sa Stemyvette garciaNo ratings yet
- Single Sphere Flows in Stokes RegimeDocument46 pagesSingle Sphere Flows in Stokes RegimeqoberifNo ratings yet
- Degradation of The Mechanical Integrity of Steam Turbine Steels Due To Stress-Corrosion Cracking in Acidic WaterDocument14 pagesDegradation of The Mechanical Integrity of Steam Turbine Steels Due To Stress-Corrosion Cracking in Acidic WaterLeila safiddineNo ratings yet
- CHEMISTRY ADVANCED LEVEL EXERCISE VOL. II REDOX REACTIONSDocument14 pagesCHEMISTRY ADVANCED LEVEL EXERCISE VOL. II REDOX REACTIONSSahil GillNo ratings yet
- CHEM 241 SyllabusDocument2 pagesCHEM 241 SyllabusBob MillerNo ratings yet
- Unit 2-Chapter 6 - Heat Treatment of MetalsDocument55 pagesUnit 2-Chapter 6 - Heat Treatment of Metalssainath reddy kesam reddyNo ratings yet
- Design Calculation SheetDocument6 pagesDesign Calculation Sheetamo3330No ratings yet
- Astm D5338Document7 pagesAstm D5338alexanderhdez100% (1)
- Evaporation FullDocument57 pagesEvaporation FullMonty KushwahaNo ratings yet
- Aci 350.4Document16 pagesAci 350.4Jason PowellNo ratings yet
- TS NEET-2019 QP Test-07 (PMTcorner - In) PDFDocument15 pagesTS NEET-2019 QP Test-07 (PMTcorner - In) PDFhuylimala0% (1)
- Mine Wastes PDFDocument18 pagesMine Wastes PDFDavidRuizNo ratings yet
- Science Form3 Chapter 9Document4 pagesScience Form3 Chapter 9Sya Myra40% (5)
- Rakesh Kumar Sonker, Kedar Singh, Rajendra Sonkawade - Smart Nanostructure Materials and Sensor Technology (2022, Springer) - Libgen - LiDocument304 pagesRakesh Kumar Sonker, Kedar Singh, Rajendra Sonkawade - Smart Nanostructure Materials and Sensor Technology (2022, Springer) - Libgen - LiDavid CaçadorNo ratings yet
- Evaluate Analytical Data AccuracyDocument15 pagesEvaluate Analytical Data AccuracyGopinathNo ratings yet
- Elemental AnalysisDocument3 pagesElemental AnalysisRicha-Lyn BeldoaNo ratings yet
- 06 Introduction To Oceanography. TomczakDocument123 pages06 Introduction To Oceanography. TomczakmishazujevNo ratings yet
- AHP 1st UnitDocument33 pagesAHP 1st UnitSith AnanthanNo ratings yet
- 10 1021@acsreagents 4191Document2 pages10 1021@acsreagents 4191Hans TorresNo ratings yet
- Experiment 7 - Heterogenous Equilibria FWR PDFDocument6 pagesExperiment 7 - Heterogenous Equilibria FWR PDFkm3197100% (1)