Professional Documents
Culture Documents
Energy Changes Worksheet
Uploaded by
Arman Serrano0 ratings0% found this document useful (0 votes)
18 views1 pageThermodynamics worksheet
Original Title
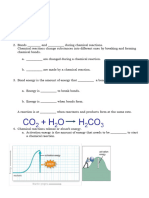
1. ENERGY CHANGES WORKSHEET
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThermodynamics worksheet
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
18 views1 pageEnergy Changes Worksheet
Uploaded by
Arman SerranoThermodynamics worksheet
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
Name: _____________________________________________ Score: ______________________________________
Grade and Section: ___________________________________ Date: _______________________________________
Energy and Chemical Change
In your textbook, read about the nature of energy.
In the space at the left, write true if the statement is true; if the statement
is false, change the italicized word or phrase to make it true.
___________________ 1. Energy is the ability to do work or produce heat.
___________________ 2. The law of conservation of energy states that energy can
be created and destroyed.
___________________ 3. Chemical potential energy is energy stored in a
substance because of its composition.
___________________ 4. Heat is a form of energy that flows from a warmer
object to a cooler object.
___________________ 5. A calorie is the amount of energy required to raise the
temperature of one gram of pure water by one degree
Celsius.
___________________ 6. A calorie is the SI unit of heat and energy.
___________________ 7. The specific heat of a substance is the amount of heat
required to raise the temperature of one gram of that
substance by one degree Celsius.
___________________ 8. Kinetic energy is energy of motion.
___________________ 9. Chemicals participating in a chemical reaction contain
only potential energy.
___________________ 10. One nutritional Calorie is equal to 100 calories.
___________________ 11. One calorie equals 4.184 joules.
___________________ 12. When a fuel is burned, some of its chemical potential
energy is lost as heat.
___________________ 13. To convert kilojoules to joules, divide the number of
kilojoules by1000 joules/1 kilojoule.
Answer the following question. Show all your work.
14. If the temperature of a 500.0-g sample of liquid water is raised 2.00°C, how much
heat is absorbed by the water? The specific heat of liquid water is 4.184
J/(g°C).
You might also like
- Study Guide Energy and Chemical Change Student EditableDocument7 pagesStudy Guide Energy and Chemical Change Student EditableRicki HanNo ratings yet
- CH 12 Study GuideDocument8 pagesCH 12 Study GuideyawahabNo ratings yet
- Work and Energy: Chapter TestDocument4 pagesWork and Energy: Chapter Testolgger vanegas100% (1)
- Heat Transfer Worksheet 14 - 15Document4 pagesHeat Transfer Worksheet 14 - 15Abu SaufaniNo ratings yet
- Sources of Heat: Form 1 Science - Unit 7.1: Heat As A Form of EnergyDocument3 pagesSources of Heat: Form 1 Science - Unit 7.1: Heat As A Form of EnergySuhaila SaniNo ratings yet
- Study Guide Thermal Energy StudentDocument4 pagesStudy Guide Thermal Energy StudentMARIAM YAMEN JAAFARNo ratings yet
- Chem181 Finals ReviewerDocument7 pagesChem181 Finals ReviewerLynne Ivy IllagaNo ratings yet
- Matter WorksheetDocument4 pagesMatter WorksheetEvelyn Cantos ZapataNo ratings yet
- Bill Nye Energy WorksheetDocument2 pagesBill Nye Energy Worksheetapi-232226389No ratings yet
- Content - Measuring HeatDocument2 pagesContent - Measuring HeatAli McDillonNo ratings yet
- Complete The SentencesDocument2 pagesComplete The SentencesAlba CubilloNo ratings yet
- Thermochemical EquationsDocument1 pageThermochemical EquationsArman SerranoNo ratings yet
- Learning Activity Sheet Grade 7 ScienceDocument6 pagesLearning Activity Sheet Grade 7 ScienceCharo Nudo PongasiNo ratings yet
- Gizmo - Conservation of Energy in A SystemDocument5 pagesGizmo - Conservation of Energy in A SystemJustin Wen33% (3)
- Bill Nye, The Science Guy! EnergyDocument2 pagesBill Nye, The Science Guy! EnergyTrevor RivardNo ratings yet
- Class 2 - Ch. 4-5 NotesDocument8 pagesClass 2 - Ch. 4-5 Notesmark chenNo ratings yet
- CH 12 Study GuideDocument8 pagesCH 12 Study Guideyawahab50% (6)
- 18.2.21 1.03.21 Reactions 2 Working From Home Booklet PART 2 AnswersDocument33 pages18.2.21 1.03.21 Reactions 2 Working From Home Booklet PART 2 Answerslokapavani_senthilNo ratings yet
- Energy Notes Video HandoutDocument4 pagesEnergy Notes Video Handoutsyd novackNo ratings yet
- ORDENADocument5 pagesORDENApepeNo ratings yet
- Chapter 2 EnergyDocument4 pagesChapter 2 EnergyMr SoupNo ratings yet
- Jack Campbell - Thermal Energy Note Sheet Assignment - 9170384Document3 pagesJack Campbell - Thermal Energy Note Sheet Assignment - 9170384JACK CAMPBELLNo ratings yet
- Endothermic and Exothermic Reaction WorksheetDocument4 pagesEndothermic and Exothermic Reaction Worksheetshashideshpande80% (5)
- Matter 1B Forms Properties and ChangesDocument49 pagesMatter 1B Forms Properties and ChangesSamKris Guerrero Malasaga67% (3)
- ChemrevDocument43 pagesChemrevgoh benNo ratings yet
- Themochemistry NotesDocument26 pagesThemochemistry NotesjayaselanNo ratings yet
- LAS - Chemistry 1 MidtermDocument27 pagesLAS - Chemistry 1 MidtermCharleneNo ratings yet
- Heat WrksheetDocument1 pageHeat Wrksheetlucerito canchanNo ratings yet
- Calor y Temperatura CorregirDocument8 pagesCalor y Temperatura CorregirMagally CastilloNo ratings yet
- Energy WebquestDocument2 pagesEnergy WebquestKlavel Pajollari100% (1)
- May Grade-7 2022Document11 pagesMay Grade-7 2022Syed Shujaat AliNo ratings yet
- Uh 1 (Remedial)Document2 pagesUh 1 (Remedial)Rahayu utamiNo ratings yet
- Activity Sheet 10 & 11 GenBio1Document6 pagesActivity Sheet 10 & 11 GenBio1Marlou GayaneloNo ratings yet
- Energy and Energy Conversions WSDocument6 pagesEnergy and Energy Conversions WScNo ratings yet
- Phase Changes SeDocument5 pagesPhase Changes Seankitjakhar350% (1)
- Physical and Chemical Changes WorksheetDocument4 pagesPhysical and Chemical Changes WorksheetAndrew ChenNo ratings yet
- P Science 6 Language Worksheets Unit 2Document4 pagesP Science 6 Language Worksheets Unit 2Nokutenda Kachere100% (1)
- B11 Organisms and Their EnvironmentDocument7 pagesB11 Organisms and Their Environmentgoh benNo ratings yet
- MatterDocument7 pagesMatterCharmaine Wright100% (1)
- Ch. 15A Thermal Energy - QuestionsDocument3 pagesCh. 15A Thermal Energy - Questionslon.bar.27No ratings yet
- Particle Model of Matter MUSA GCSEDocument16 pagesParticle Model of Matter MUSA GCSEMethyl OrangeNo ratings yet
- I. IdentificationDocument1 pageI. IdentificationRoselyn LoraNo ratings yet
- Student Exploration: Collision TheoryDocument7 pagesStudent Exploration: Collision TheoryNicholas CrowellNo ratings yet
- F3 SN CHP 5 2023Document24 pagesF3 SN CHP 5 2023NG YI ZHI MoeNo ratings yet
- Science Review - Energy Name: - Date: - AnswersDocument3 pagesScience Review - Energy Name: - Date: - AnswersSarmauli SibaraniNo ratings yet
- Module 4 Technical WritingDocument4 pagesModule 4 Technical WritingSJ AjineNo ratings yet
- Week 6Document6 pagesWeek 6amiillysacalinisanNo ratings yet
- Ch.9-Study Guide Chemical Reactions Teacher EditableDocument10 pagesCh.9-Study Guide Chemical Reactions Teacher EditableOrganize gnqNo ratings yet
- ThermodynamicsDocument2 pagesThermodynamicsKristian Jay NantaNo ratings yet
- Quiz 1 Chemistry/Physics/BiologyDocument7 pagesQuiz 1 Chemistry/Physics/Biologyeric sivaneshNo ratings yet
- PSCH 0814 KeyDocument2 pagesPSCH 0814 KeyJan Ira RenolayanNo ratings yet
- Chapter 4: Heat: 4.1 Understanding Thermal EquilibriumDocument34 pagesChapter 4: Heat: 4.1 Understanding Thermal EquilibriumshazillaNo ratings yet
- Maliyah Winston - Vocabulary Energy TransformationDocument2 pagesMaliyah Winston - Vocabulary Energy Transformationkaty collins0% (1)
- Energy Transformation Virtual LabDocument4 pagesEnergy Transformation Virtual LabJerome CarterNo ratings yet
- 6/7 Grade Science Study Guide: Energy: Class: - NameDocument2 pages6/7 Grade Science Study Guide: Energy: Class: - Nameapi-404761117No ratings yet
- Book 3 - Chemical Equations Balancing Work Sheet ChemistryDocument16 pagesBook 3 - Chemical Equations Balancing Work Sheet ChemistryAzain Cardenas100% (1)
- Endothermic and Exothermic Reaction WorksheetDocument4 pagesEndothermic and Exothermic Reaction WorksheetNubar Mammadova0% (1)
- Chapter+2 4-5+Guided+NotesDocument3 pagesChapter+2 4-5+Guided+Noteskparsons938512No ratings yet
- Science 6: Learning Competency: Demonstrate How Sound, Heat, Light and Electricity Can Be TransformedDocument5 pagesScience 6: Learning Competency: Demonstrate How Sound, Heat, Light and Electricity Can Be TransformedRONA FLESTADONo ratings yet
- States of Matter & Phase Equilibria: Essential Chemistry Self-Teaching GuideFrom EverandStates of Matter & Phase Equilibria: Essential Chemistry Self-Teaching GuideNo ratings yet
- THERMOCHEMISTRYDocument75 pagesTHERMOCHEMISTRYArman SerranoNo ratings yet
- THERMOCHEMISTRYDocument75 pagesTHERMOCHEMISTRYArman SerranoNo ratings yet
- Reaction SpontaneityDocument1 pageReaction SpontaneityArman SerranoNo ratings yet
- QR Driven Strategy WorkflowDocument2 pagesQR Driven Strategy WorkflowArman SerranoNo ratings yet
- Reaction SpontaneityDocument1 pageReaction SpontaneityArman SerranoNo ratings yet
- THERMOCHEMISTRYDocument75 pagesTHERMOCHEMISTRYArman SerranoNo ratings yet
- Thermochemical EquationsDocument1 pageThermochemical EquationsArman SerranoNo ratings yet
- Heat in Chemical Processes LessonDocument8 pagesHeat in Chemical Processes LessonArman SerranoNo ratings yet
- Research Golden SnailDocument39 pagesResearch Golden SnailArman SerranoNo ratings yet
- Quiz Gen Chem ImfaDocument1 pageQuiz Gen Chem ImfaArman SerranoNo ratings yet