Professional Documents
Culture Documents
Titration Worksheet
Uploaded by
p berger0 ratings0% found this document useful (0 votes)
320 views1 pageThe document contains 5 chemistry problems involving the titration of acids and bases to determine molar concentrations. Specifically, it involves using the moles of acid and base reacted in a titration, along with their volumes, to calculate the concentration of the unknown substance. The problems cover titrating sulfuric acid, hydrochloric acid, and nitric acid solutions of unknown concentration against sodium hydroxide and potassium hydroxide solutions of known molarity.
Original Description:
Original Title
Titration worksheet
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document contains 5 chemistry problems involving the titration of acids and bases to determine molar concentrations. Specifically, it involves using the moles of acid and base reacted in a titration, along with their volumes, to calculate the concentration of the unknown substance. The problems cover titrating sulfuric acid, hydrochloric acid, and nitric acid solutions of unknown concentration against sodium hydroxide and potassium hydroxide solutions of known molarity.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
320 views1 pageTitration Worksheet
Uploaded by
p bergerThe document contains 5 chemistry problems involving the titration of acids and bases to determine molar concentrations. Specifically, it involves using the moles of acid and base reacted in a titration, along with their volumes, to calculate the concentration of the unknown substance. The problems cover titrating sulfuric acid, hydrochloric acid, and nitric acid solutions of unknown concentration against sodium hydroxide and potassium hydroxide solutions of known molarity.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
1.
In a titration, 20 cm3 of 0·5 mol/dm3 sodium hydroxide is
exactly neutralised by 40 cm3 of sulfuric acid. What is
the concentration of the sulfuric acid?
2. It takes 38 mL of 0.75 M NaOH solution to completely
neutralize 155 mL of a sulfuric acid solution (H2SO4). What is
the concentration of the H2SO4 solution?
3. It takes 12.5 mL of a 0.30 M HCl solution to neutralize 285
mL of NaOH solution. What is the concentration of the NaOH
solution?
4. A titration of a 25.00 mL sample of a hydrochloric acid
solution of unknown molarity reaches the equivalence point
when 38.28 mL of 0.4370 M KOH solution has been added.
What is the molarity (molar concentration) of the HCl
solution?
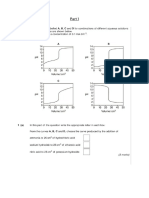
5.
Concentration of Nitric Acid ( HNO3) was 1.2 mol dm-3. The
pipetted volume was 25 cm3. Use the table to find the molar
concentration of NaOH.
You might also like
- CAPE Chemistry Module 1 - MolesDocument4 pagesCAPE Chemistry Module 1 - Molesp bergerNo ratings yet
- Chlorine Cape Chem Unit 2 Mod 3Document25 pagesChlorine Cape Chem Unit 2 Mod 3p bergerNo ratings yet
- Chlorine Cape Chem Unit 2 Mod 3Document25 pagesChlorine Cape Chem Unit 2 Mod 3p bergerNo ratings yet
- Fd11a - Caribbean - Cultural - ExpressionDocument42 pagesFd11a - Caribbean - Cultural - Expressionp bergerNo ratings yet
- Fd11a - Caribbean - Cultural - ExpressionDocument42 pagesFd11a - Caribbean - Cultural - Expressionp bergerNo ratings yet
- Buffers and PHDocument29 pagesBuffers and PHp bergerNo ratings yet
- Objective: Write A Formula Equation, Complete Ionic Equation, and Net Ionic Equation That Represent A ReactionDocument8 pagesObjective: Write A Formula Equation, Complete Ionic Equation, and Net Ionic Equation That Represent A Reactionp bergerNo ratings yet
- P&D Iron in Iron SupplementsDocument3 pagesP&D Iron in Iron Supplementsp berger100% (1)
- Electrolyte WorksheetDocument1 pageElectrolyte WorksheetAde JayusNo ratings yet
- Redox and Acid-Base Titration CalculationsDocument9 pagesRedox and Acid-Base Titration Calculationsemily_liu_5No ratings yet
- Exercises For The Complexometric Titrati PDFDocument1 pageExercises For The Complexometric Titrati PDFDarwin Castellanos100% (1)
- Exp4 Analytical ChemistryDocument4 pagesExp4 Analytical ChemistryThulileLimama0% (1)
- Pku 2018 Analitik IV Era 085Document10 pagesPku 2018 Analitik IV Era 085Era MelaniaNo ratings yet
- Organic Benzyl AcetateDocument4 pagesOrganic Benzyl Acetatemoaz ahmadNo ratings yet
- Tutorial Complexometric TitrationDocument2 pagesTutorial Complexometric TitrationCeyah NurrNo ratings yet
- Shapes of Complexes of Transition MetalsDocument9 pagesShapes of Complexes of Transition MetalsDonlee CastelloNo ratings yet
- Tutorial 1Document2 pagesTutorial 1Tevin TK KrishnaNo ratings yet
- MSDS For CDEA PDFDocument5 pagesMSDS For CDEA PDFsanjay ukalkar100% (1)
- Salt Hydrolysis: SRCL No Ba (Po) CusoDocument2 pagesSalt Hydrolysis: SRCL No Ba (Po) CusoDevon100% (1)
- Calculating equilibrium concentrations and mass of products in chemical reactionsDocument5 pagesCalculating equilibrium concentrations and mass of products in chemical reactionsSalsaLinaSinasaNo ratings yet
- C NMR Spectroscopy Worksheet (30 Points) Due 2/24/11 in LectureDocument4 pagesC NMR Spectroscopy Worksheet (30 Points) Due 2/24/11 in LectureNurillahi Febria LeswanaNo ratings yet
- Buffer SolutionsDocument19 pagesBuffer SolutionsMuskaan BindalNo ratings yet
- Some Basic Concepts of ChemistryDocument12 pagesSome Basic Concepts of ChemistryNikhil BhattNo ratings yet
- CH 17Document54 pagesCH 17firebot4No ratings yet
- CBT CHM 116 Exam-3Document17 pagesCBT CHM 116 Exam-3Martins A. ThobiNo ratings yet
- Identifikasi Cairan Organik Berdasarkan Sifat FisiknyaDocument14 pagesIdentifikasi Cairan Organik Berdasarkan Sifat FisiknyaAnggraini WidyaNo ratings yet
- OXAZOLEthiazolimidazoleDocument7 pagesOXAZOLEthiazolimidazole아미르No ratings yet
- Coordination Compounds Multiple C. QuesDocument6 pagesCoordination Compounds Multiple C. QuesShivam KumarNo ratings yet
- Latihan Soal GasDocument1 pageLatihan Soal GasnajmahsNo ratings yet
- The Report Result of Experiment Hess LawDocument17 pagesThe Report Result of Experiment Hess LawFairoozAnwar67% (3)
- Chapter 1Document5 pagesChapter 1Christian EduardoNo ratings yet
- Chemistry Worksheet - Organic CompoundsDocument1 pageChemistry Worksheet - Organic CompoundsBabtista EzraNo ratings yet
- Asam BasaDocument7 pagesAsam BasaAmanah Uluputty0% (1)
- Chemistry - Xii: Coordination Compounds - NomenclatureDocument2 pagesChemistry - Xii: Coordination Compounds - NomenclatureManoj Gupta100% (1)
- Qaulitative AnalysisDocument65 pagesQaulitative AnalysisAhmed AwadNo ratings yet
- Stoikiometri (Stoichiometry) : Perhitungan Kimia (Chemical Calculation)Document12 pagesStoikiometri (Stoichiometry) : Perhitungan Kimia (Chemical Calculation)Bakhitah NurulNo ratings yet
- Health and Safety of BenzeneDocument6 pagesHealth and Safety of BenzeneYeniNo ratings yet
- ASV ANALYSIS OF METALSDocument32 pagesASV ANALYSIS OF METALSChitrakshi GoelNo ratings yet
- Q NmrH1highresDocument5 pagesQ NmrH1highresKhondokar TarakkyNo ratings yet
- Pembuatan Cis Dan Trans Kalium DioksalatodiakuokromatDocument10 pagesPembuatan Cis Dan Trans Kalium DioksalatodiakuokromatZulvana Anggraeni HarvianNo ratings yet
- Msds Natrium Oksalat PDFDocument6 pagesMsds Natrium Oksalat PDFrilmaNo ratings yet
- Material de Apoyo Reading ChemistryDocument7 pagesMaterial de Apoyo Reading ChemistryJohan GallegoNo ratings yet
- CHE 123 HWK Back and Redox TitrationsDocument3 pagesCHE 123 HWK Back and Redox TitrationsJuiloNo ratings yet
- S7 11012021 Acid Base Titrations WS With ANSWERSDocument7 pagesS7 11012021 Acid Base Titrations WS With ANSWERSFatima Ahmed-VeriterNo ratings yet
- Pembuatan Sabun Cair Dari Minyak JelantaDocument5 pagesPembuatan Sabun Cair Dari Minyak JelantaOjan KentNo ratings yet
- CH2.2 - AlkeneDocument48 pagesCH2.2 - AlkeneNur Ain SyuhadaNo ratings yet
- 練習單3 2Document8 pages練習單3 2Marco RezendeNo ratings yet
- Ferrous Ammonium Sulfate HexahydrateDocument6 pagesFerrous Ammonium Sulfate Hexahydrateyowis mbokahNo ratings yet
- Determination of Sodium Sulphate Purity by Gravimetric AnalysisDocument4 pagesDetermination of Sodium Sulphate Purity by Gravimetric AnalysisNazmul hasanNo ratings yet
- Making Double SaltsDocument3 pagesMaking Double SaltssesamproNo ratings yet
- Survey Dan Ringkasan Sebuah Nomenklatur Untuk Restriksi Enzim, DNA Methyltransferases, HomingDocument16 pagesSurvey Dan Ringkasan Sebuah Nomenklatur Untuk Restriksi Enzim, DNA Methyltransferases, Homingalief utamaNo ratings yet
- CHM 423 PDFDocument110 pagesCHM 423 PDFKokoh EmmanuelNo ratings yet
- PS3 - 12 30 1 30orcdmmnDocument16 pagesPS3 - 12 30 1 30orcdmmnMarianne Camille de GuzmanNo ratings yet
- Title: K (Cu (C O) ) .2H ODocument10 pagesTitle: K (Cu (C O) ) .2H ObabeNo ratings yet
- Report of ElectrogravimetryDocument12 pagesReport of ElectrogravimetryKrisna Raditya PNo ratings yet
- International JournalDocument4 pagesInternational JournalBrahma Hakim Yuanda HutabaratNo ratings yet
- Effect of Surface Area On The Rate of ReactionDocument10 pagesEffect of Surface Area On The Rate of ReactionsuffyandaimNo ratings yet
- Mind Map ChemistryDocument3 pagesMind Map ChemistryTheesha SophieNo ratings yet
- AS Chemistry Topic 3 MCQs on Chemical BondingDocument6 pagesAS Chemistry Topic 3 MCQs on Chemical BondingAijaz AhmedNo ratings yet
- Chemical Engineering Calculations Ii (TKK 1319 - 2 SKS) : Meta Fitri Rizkiana, S.T., M.Sc. NRP. 760017111Document20 pagesChemical Engineering Calculations Ii (TKK 1319 - 2 SKS) : Meta Fitri Rizkiana, S.T., M.Sc. NRP. 760017111Riatus SNo ratings yet
- CHEM 1235: MgO & CaCO3 NeutralizationDocument1 pageCHEM 1235: MgO & CaCO3 NeutralizationJesseca Calaunan QuintoNo ratings yet
- Students' Misconceptions in Electrochemistry Current Flow in Electrolyte Solution PDFDocument5 pagesStudents' Misconceptions in Electrochemistry Current Flow in Electrolyte Solution PDFJordon Alvarado100% (1)
- Acid Base Titration ExplainedDocument31 pagesAcid Base Titration ExplainedMuhammad Tariq RazaNo ratings yet
- Neutralization Calculations and TitrationsDocument3 pagesNeutralization Calculations and TitrationsDianne Kate CadioganNo ratings yet
- Titration Practice Worksheet: SCH 3uoDocument1 pageTitration Practice Worksheet: SCH 3uohan thiNo ratings yet
- Titrations Practice ProblemsDocument3 pagesTitrations Practice ProblemsbenitonziratimaanaNo ratings yet
- Guidelines For Online Learning 2Document1 pageGuidelines For Online Learning 2p bergerNo ratings yet
- Saponification: Esters, Soapless and Soapy DetergentsDocument17 pagesSaponification: Esters, Soapless and Soapy Detergentsp bergerNo ratings yet
- CXC Release of TopicsDocument50 pagesCXC Release of TopicsJacob seraphineNo ratings yet
- The Caribbean in Motion: Emancipation, Migration and East Indian IndentureshipDocument38 pagesThe Caribbean in Motion: Emancipation, Migration and East Indian Indentureshipp bergerNo ratings yet
- FD11A - Religion and Social Control in The Caribbean8Document37 pagesFD11A - Religion and Social Control in The Caribbean8p bergerNo ratings yet
- Titration WorksheetDocument1 pageTitration Worksheetp bergerNo ratings yet
- Mod 1 - Sect 3 - MolesDocument4 pagesMod 1 - Sect 3 - Molesp bergerNo ratings yet
- Chemistry UNIT 1 MOD 1 AssignmentDocument1 pageChemistry UNIT 1 MOD 1 Assignmentp bergerNo ratings yet
- SBA #6 Title: Rate of Reaction Aim: (A) To Investigate The Effect of Concentration On The Rate of Reaction Between Solid MagnesiumDocument2 pagesSBA #6 Title: Rate of Reaction Aim: (A) To Investigate The Effect of Concentration On The Rate of Reaction Between Solid Magnesiump berger100% (1)
- Oxidation - ReductionDocument30 pagesOxidation - Reductionp bergerNo ratings yet
- SBA - Ligand ExchangeDocument2 pagesSBA - Ligand Exchangep berger100% (1)
- Atomic Theory and The AtomDocument44 pagesAtomic Theory and The Atomp berger100% (1)