0% found this document useful (0 votes)
339 views5 pagesColumn Agglutination Technology: The Antiglobulin Test: Agglutination of Red Cells
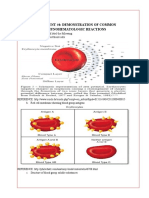
This document describes a new method called column agglutination technology (CAT) for typing and screening blood. CAT uses glass bead microcolumns to separate agglutinated red blood cells from unagglutinated cells during centrifugation. Two CAT anti-human globulin tests were developed containing rabbit antibodies to detect IgG- or complement-sensitized red cells. The CAT tests were compared to conventional tube tests on 228 samples, showing 94-97% agreement. CAT eliminates the need to wash red cells and provides a more objective and easier to perform test compared to tube and microplate methods.
Uploaded by
OB3 Ortho Clinical DiagnosticsCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
339 views5 pagesColumn Agglutination Technology: The Antiglobulin Test: Agglutination of Red Cells
This document describes a new method called column agglutination technology (CAT) for typing and screening blood. CAT uses glass bead microcolumns to separate agglutinated red blood cells from unagglutinated cells during centrifugation. Two CAT anti-human globulin tests were developed containing rabbit antibodies to detect IgG- or complement-sensitized red cells. The CAT tests were compared to conventional tube tests on 228 samples, showing 94-97% agreement. CAT eliminates the need to wash red cells and provides a more objective and easier to perform test compared to tube and microplate methods.
Uploaded by
OB3 Ortho Clinical DiagnosticsCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd