Professional Documents
Culture Documents
DR Sajida Sultana PDF
DR Sajida Sultana PDF
Uploaded by
Sara Qutubuddin0 ratings0% found this document useful (0 votes)
34 views1 pageThis document is a COVID-19 test result from the National Institute of Virology at the University of Karachi for a 56-year-old female named Dr. Sajida Sultana. The test was for SARS-CoV-2 RNA in a nasopharyngeal/oropharyngeal swab sample, and the result was negative. The test was performed using real-time PCR with controls and had an analytical sensitivity of 0.58 copies/μL.
Original Description:
Original Title
Dr Sajida Sultana.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document is a COVID-19 test result from the National Institute of Virology at the University of Karachi for a 56-year-old female named Dr. Sajida Sultana. The test was for SARS-CoV-2 RNA in a nasopharyngeal/oropharyngeal swab sample, and the result was negative. The test was performed using real-time PCR with controls and had an analytical sensitivity of 0.58 copies/μL.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
34 views1 pageDR Sajida Sultana PDF
DR Sajida Sultana PDF
Uploaded by
Sara QutubuddinThis document is a COVID-19 test result from the National Institute of Virology at the University of Karachi for a 56-year-old female named Dr. Sajida Sultana. The test was for SARS-CoV-2 RNA in a nasopharyngeal/oropharyngeal swab sample, and the result was negative. The test was performed using real-time PCR with controls and had an analytical sensitivity of 0.58 copies/μL.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
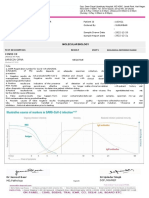
National Institute of Virology
Dr. Panjwani Center For Molecular Medicine and Drug Research
International Center For Chemical and Biological Sciences
University of Karachi
Name : Dr Sajida Sultana S/O, W/O, D/O : Qutubddin CNIC : 4220183777168
Age / Gender : 56 / Female MR / Lab No : 7248 Sample Received : 20.07.2020
Reporting Date : 21.07.2020 Referred By : Const. Virologist : Dr. Muhammad Rashid
Test Name : SARS-COV-2 (COVID-19) RNA DETECTION BY PCR (QUALITATIVE)
Sample : Nasopharyngeal/Oropharyngeal Swab
Result : SARS-COV-2 RNA (COVID-19) Negative
Principle : The Real-Time PCR Coronavirus (COVID-19) assay is an in vitro diagnostic test based on
fluorescence-based detection of specific SARS-CoV-2 targets in the viral genome.
Method:
The test was performed after RNA extraction using automated RNA extraction machines and amplified using
real-time PCR machines (BioRad CFX-96/ABI QuantStudio 5). The following controls were included: RNA
extraction control, PCR positive, and negative controls.
The analytical sensitivity of this assay is 0.58 copies/µL.
Electronically verified on 21.07.2020 03:41 PM No Signature required.
You might also like
- Yasser M. Bin KhalidDocument1 pageYasser M. Bin KhalidYasser KhalidNo ratings yet
- T2200053158 P2200045869 0 T2200053158 62 0 20000115 $ml-DefaultDocument1 pageT2200053158 P2200045869 0 T2200053158 62 0 20000115 $ml-DefaultShaira BungayNo ratings yet
- Aed2020-27745 MR - Tejashwin Ravishankar 129334Document1 pageAed2020-27745 MR - Tejashwin Ravishankar 129334sadhanaNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument4 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range Methodsourabhshrivastava80No ratings yet
- Department of Molecular Biology and Cytogenetics:: Mr. Banavathu Gopi Kiran NaikDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Mr. Banavathu Gopi Kiran NaikGopi Kiran NaikNo ratings yet
- Mangal Cook ReportDocument2 pagesMangal Cook ReportSanjeev SharmaNo ratings yet
- Laboratory Investigation Report: 32 Years/M 1222956254Document1 pageLaboratory Investigation Report: 32 Years/M 1222956254Chaminda HiroshanNo ratings yet
- SMSHLD tPEkEzDocument1 pageSMSHLD tPEkEzPranjal JindalNo ratings yet
- Shaheed Mohta Shaheed Mohtarma Benazir Bhutto Lab Lyari Lab LyariDocument1 pageShaheed Mohta Shaheed Mohtarma Benazir Bhutto Lab Lyari Lab LyariMuhammad TahirNo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna CT Value of Confirmatory Gene: Target (S)Document2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna CT Value of Confirmatory Gene: Target (S)Ankit SuraNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinessssNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesMohammed Shafi CpNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesMohammed Shafi CpNo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna CT Value of Confirmatory Gene: Target (S)Document2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna CT Value of Confirmatory Gene: Target (S)Ankit SuraNo ratings yet
- Molecular Laboratory Test Result: de Loreto, San Isidro, City of Parañaque, NCR, Fourth District (Not A Province)Document2 pagesMolecular Laboratory Test Result: de Loreto, San Isidro, City of Parañaque, NCR, Fourth District (Not A Province)JJS INTERNATIONAL PLACEMENT AGENCY COMPANYNo ratings yet
- Rahul SharmaDocument3 pagesRahul Sharmaarunitsaraogi7No ratings yet
- Jinnah Central Lab: Jinnah Hospital & AIMC Lahore, PakistanDocument2 pagesJinnah Central Lab: Jinnah Hospital & AIMC Lahore, Pakistanafshan liaqatNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodGirija Prasad SwainNo ratings yet
- CGH202008000915 - Lab A2 2020 2231 - Laboratory - Covid PCR Test PDFDocument2 pagesCGH202008000915 - Lab A2 2020 2231 - Laboratory - Covid PCR Test PDFMichael JonasanNo ratings yet
- Interpretation Notes: Interpretation NotesDocument1 pageInterpretation Notes: Interpretation Notesadish narayanNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesMohammed Shafi CpNo ratings yet
- This Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureDocument1 pageThis Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureBianca Alana Hizon LimjucoNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodShravan RajavathNo ratings yet
- Covid ReportDocument5 pagesCovid Reportraojip1232No ratings yet
- RTPCRDocument1 pageRTPCRVouch Pro AdminNo ratings yet
- ICMRDocument1 pageICMRP.Hari PrasadNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodPritam JanaNo ratings yet
- Laboratory Report:: MR - Rohan Dhawa Name: P508466 Patient IDDocument1 pageLaboratory Report:: MR - Rohan Dhawa Name: P508466 Patient IDRohan DhawaNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDevi Sri PrasadNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodLIKE IT BRONo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)Document2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)manwanimuki12No ratings yet
- Madhan - 642161200148401 2Document2 pagesMadhan - 642161200148401 2madhanNo ratings yet
- Test Report: MR - DURAI RAJ (77/M)Document2 pagesTest Report: MR - DURAI RAJ (77/M)vijay singhNo ratings yet
- Patient ReportDocument2 pagesPatient ReportVeeraj SinghNo ratings yet
- This Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureDocument1 pageThis Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureRyan FernandezNo ratings yet
- ReportDocument1 pageReportTahsheen sarwarNo ratings yet
- Divya Bangera MBBS, MD Microbiology MME Team LeadDocument2 pagesDivya Bangera MBBS, MD Microbiology MME Team LeadRajavardhanNo ratings yet
- ArvindDocument2 pagesArvindSukhmeet SinghNo ratings yet
- Department of Molecular Biology Covid-19 Virus Qualitative PCRDocument2 pagesDepartment of Molecular Biology Covid-19 Virus Qualitative PCRpooja sharmaNo ratings yet
- DownloadDocument1 pageDownloadSAI SHARANNo ratings yet
- Raghbir Chand MukhtiaraDocument1 pageRaghbir Chand MukhtiaraBharath YemireddyNo ratings yet
- MR RishadDocument1 pageMR RishadGdhdud DbdhudNo ratings yet
- Sargam SoodDocument1 pageSargam SoodMayank JunejaNo ratings yet
- Molecular Biology Report: Test Result MethodologyDocument1 pageMolecular Biology Report: Test Result MethodologyMohamedNo ratings yet
- TestReport - 22 06 2021 - Apollo 2471624375836407Document2 pagesTestReport - 22 06 2021 - Apollo 2471624375836407thakuryaNo ratings yet
- T2100132822 P2100112050 0 T2100132822 Telecare 0 19811126 $ml-DefaultDocument1 pageT2100132822 P2100112050 0 T2100132822 Telecare 0 19811126 $ml-DefaultRoyzen VillaruelNo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna CT Value of Confirmatory Gene: Target (S)Document2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna CT Value of Confirmatory Gene: Target (S)Ankit SuraNo ratings yet
- Sars-Cov2 (Covid-19) Real Time RT PCR Test: Positive and Negative Controls For All The Three Genes Were SatisfactoryDocument1 pageSars-Cov2 (Covid-19) Real Time RT PCR Test: Positive and Negative Controls For All The Three Genes Were Satisfactorygowtham thakutNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Mr. Machineni Sai KrishnaDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Mr. Machineni Sai KrishnaVenkat Sai Dhilli Engg. 2020No ratings yet
- Sajal AgarwalDocument1 pageSajal AgarwalMayank JunejaNo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)Document2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)manwanimuki12No ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodRutuja ShindeNo ratings yet
- Sars-Cov-2 RT PCR Testing: Test Description Method ResultDocument1 pageSars-Cov-2 RT PCR Testing: Test Description Method ResultMAYUR PATELNo ratings yet
- Department of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodanilsgaikwadNo ratings yet
- Sars-Cov-2: Empowers To Live WellDocument2 pagesSars-Cov-2: Empowers To Live WellAkhil KNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodRahul SankaranNo ratings yet
- MR - AJINKYA KASAR LabReportNew-4Document2 pagesMR - AJINKYA KASAR LabReportNew-4Ajinkya kasarNo ratings yet
- Rahul Test ReportDocument1 pageRahul Test ReportNikHilPaTilNo ratings yet
- ReportDocument2 pagesReportHarish KumsrNo ratings yet
- Liquid Biopsy: New Challenges in the era of Immunotherapy and Precision OncologyFrom EverandLiquid Biopsy: New Challenges in the era of Immunotherapy and Precision OncologyAntonio RussoNo ratings yet