Professional Documents
Culture Documents
Topic 6 Air Combustion, Rusting and Fire Fighting.
Uploaded by
Trump DonaldOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Topic 6 Air Combustion, Rusting and Fire Fighting.
Uploaded by
Trump DonaldCopyright:
Available Formats
TOPIC 6: AIR COMBUSTION, RUSTING AND FIRE FIGHTING.
Composition of air
Air is a mixture of different gases. The gases that make up the air include nitrogen, oxygen,
carbon dioxide, noble gases (argon, helium, neon, krypton and xenon) and a little water vapour.
Air may also contain traces of impurities such as carbon monoxide (CO), sulphur dioxide (SO 2),
hydrogen sulphide (H2S) and other gases. The presence of these gases in air results in air
pollution. Table bellow shows the composition of air by volume. The proportion of water vapour
and impurities in air is very variable.
The Gases Present in Air and their Proportions
Name the gases present in air and their proportions
The composition of air is not exactly the same everywhere. It changes slightly from day to day
and from place to place. There is more water vapour in the air on a damp day and in air above
water bodies such as oceans, seas, lakes, rivers, etc. Over busy cities and industrial areas there is
more carbon dioxide. But the uneven heating of the earth's surface by the sun causes the air to
move continually, resulting in winds. The resultant winds spread the pollutants around.
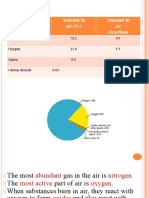
The percentage composition of air by volume
Gas Approximate percentage
Nitrogen 78.00%
Oxygen 21.00%
Noble (rare) gases mainly argon 0.94%
Carbon dioxide 0.03%
Water vapour 0 – 4%
The Presence of Different Gases in Air
Demonstrate the presence of different gases in air
The determination of air by mass was carried out by Dumas in 1841. The apparatus used
consists of three units as shown bellow.
The three parts of the apparatus include the following:
Determination of the composition of air by weight
1. Several U-tubes containing potassium hydroxide pellets to remove carbon dioxide (only
one tube shown in the figure for simplicity).
PREPARED BY MACDONALD RICHARD
2. Another set of U-tubes containing concentrated sulphuric acid to remove water vapour
(only one tube shown in the figure).
3. A heated, weighed glass tube containing finely divided copper to absorb oxygen.
The three parts of the apparatus would, therefore, remove all carbon dioxide, water vapour and
oxygen contained in air. The remaining gas which enters the weighed evacuated flask (globe)
will be atmospheric nitrogen and, of course, plus the rare gases. The copper will have reacted
with all oxygen to form copper (II) oxide. The increase in mass of the copper will give the mass
of oxygen. The increase in weight of the globe will be due to the weight of nitrogen and the rare
gases. If we neglect the weight of carbon dioxide, the percentage of oxygen by mass (weight) in
dry, pure air is 23.2% and the remaining 76.8% is the percentage of nitrogen and rare gases.
The presence of nitrogen in air
In order to demonstrate the presence of nitrogen in air, we need to carry out an experiment that
will convert the nitrogen of the air into a chemically recognizable substance. This is easily done
by strongly heating magnesium in the residual gas from the above experiment. Magnesium and
nitrogen will react thus:
Upon treatment with water, magnesium nitrite gives ammonia gas. The gas can be recognized by
its characteristic smell and its action of turning red litmus paper to blue.
The presence of oxygen in air
Oxygen is known as the active portion of the air because it supports combustion and combines
with many other substances. Its presence and composition in air can be determined by using
these properties. Any of the following two (2) experiments can be used to determine the
composition, by volume of oxygen contained in air.
The Percentage of Oxygen in Air Experimentally
Determine the percentage of oxygen in air experimentally
1. Experiment. Determination of the presence and proportion of oxygen in air by
combustion of a candle
Method
1. Place a small candle on a plastic lid or any object that can float. Then set up the apparatus
as shown in figure bellow. Sodium hydroxide is used in order to absorb the carbon dioxide
gas produced by a burning candle.
2. Light the candle and place the measuring cylinder over the top. Note the level of sodium
hydroxide solution in the measuring cylinder at the start. A candle will stop burning (go
off) once all the oxygen in the cylinder is used up.
3. When the candle goes off, leave the apparatus to cool to room temperature. The purpose of
cooling is to let the heated and expanded air to return to its normal condition. Then note the
level of sodium hydroxide solution in the measuring cylinder.
.
PREPARED BY MACDONALD RICHARD
Determining the presence and percentage composition of oxygen in air by burning a candle
Observation and findings
The oxygen in air enclosed in the measuring cylinder is used to burn the candle to produce
carbon dioxide gas. The carbon dioxide so produced dissolves in sodium hydroxide solution.
The dissolved carbon dioxide causes the level of sodium hydroxide solution to rise up. The
oxygen gas used to burn the candle is practically equal to the amount of carbon dioxide
produced. This fact is, therefore, used to calculate the percentage of oxygen in air.
Model results
In the experiment, the initial volume of air was found to be 70.5 cm3 and the final volume was
55 cm3. The percentage of oxygen in the air is calculated in two steps:
1. To find the volume of oxygen used up to burn the candle (which is practically equal to the
volume of carbon dioxide produced and then absorbed by sodium hydroxide), we subtract the
final volume of air from the initial volume
Volume or oxygen used = Initial volume of air – final volume of air
Therefore, the volume of oxygen used for combustion of the candle = 14.7 cm.
Alternatively, the volume of oxygen used up can be calculated by subtracting the initial volume
of sodium hydroxide solution from the final volume. That is: Volume of oxygen used = final
volume of sodium hydroxide – initial volume of sodium hydroxide = Volume of carbon dioxide
dissolved in sodium hydroxide.
Therefore, the percentage of oxygen =
In practice, it is difficult to get an accurate result with the above experiment.
This is due to a number of reasons such as:
PREPARED BY MACDONALD RICHARD
1. Not all the carbon dioxide is absorbed by the sodium hydroxide.
2. The candle may go out (stop burning) before all the oxygen is used up due to accumulation
of carbon dioxide in the cylinder.
3. The heating of the air inside the measuring cylinder causes the gases to expand. This is why
it is essential that the gases be allowed to cool to room temperature before reading the
level.
Experiment with combustion of copper in air gives the more accurate results than the
combustion of the candle. The copper reacts with oxygen in the air to give copper (II) oxide.
2. Experiment . Determination of the presence and proportion of oxygen in air by the
combustion of copper in air
Method
1. Set up the apparatus as shown in figure bellow. Syringe A should contain 100 cm of air,
syringe B should be empty.
2. Heat the copper strongly and pass the air from syringe A back and forth (by pushing the
piston of the syringe inward and outward) over the copper turnings a few times. Allow the
air to cool and measure the volume of air in syringe A.
3. Repeat the heating and cooling until the volume of air that remains in syringe A is constant.
The copper is heated and cooled several times to ensure that it reacts with all oxygen in the
sample of air.
`
Determining the presence and percentage composition of oxygen in air by heating copper
Observations and findings
2. The final volume of air in the syringe, at the end of the experiment, is less than that of the
original volume. This is because oxygen in the original air has combined with copper
Model of result
The volume of air in the syringe at different heating and cooling is as shown below:
Initial volume before heating = 100
Volume after first heating and cooling = 82
Volume after third heating and cooling = 79
PREPARED BY MACDONALD RICHARD
The volume of oxygen used up = Initial volume of air before cooling - volume of air after the
last heating and cooling
= 100 - 79
= 21
The presence of carbon dioxide in air
Carbon dioxide is present in air to the extent of 0.03% by volume. The gas is formed during the
combustion of all common fuels – wood, coal, coke, natural gas, petrol, diesel, paraffin oil, etc,
all of which contain carbon.
It is breathed out as a waste product of respiration by all animals. All sorts of combustion and
burning produce carbon dioxide. The gas produced by all these processes accumulates in air.
However, the amount of carbon dioxide in air remains constant instead of the tremendous
quantities released into the atmosphere. This is because plants take up carbon dioxide. They then
convert it into complex starchy compounds during photosynthesis. The gas also dissolves in
ocean water and other water bodies.
The presence of carbon dioxide in air can be shown by passing air through a test tube containing
some limewater (figure 6.5). After a time, the limewater turns milky. This shows the presence of
carbon dioxide.
The reaction involved is as follows:
``
Testing for the presence of carbon dioxide in air
The presence of water vapour in air
PREPARED BY MACDONALD RICHARD
Water vapour is present in air in varying quantities. It is given off by evaporation from the
oceans, lakes and rivers. The presence of water vapour in air can be demonstrated by exposing
deliquescent substances to the air on a watch glass. These are substances which when exposed to
air tend to absorb much moisture from the air, dissolve in that moisture, and finally form a
solution. Examples of deliquescent substances include calcium chloride, sodium hydroxide and
phosphorous pentoxide.
The resulting solution is distilled. The colourless liquid obtained from distillation may be proved
to be water by various water tests such as use of cobalt chloride paper or anhydrous copper (II)
sulphate. The cobalt chloride paper turns from blue to pink in the presence of water. The white
anhydrous copper (II) sulphate turns blue. Any of the two tests confirms the presence of water.
Alternatively, one may expose the anhydrous copper (II) sulphate salt to open air straight away
for quite some time and then observe any change in its colour and/or form. Upon absorption of
water vapour from the air, the white, powdery and anhydrous copper sulphate salt turns into
hydrated blue crystals.
The noble (rare) gases
About 1% of the air by volume is made up of the noble gases. The most abundant of the noble
gases is argon. Others are neon, xenon, krypton and helium. The proportion of these four is very
minute. Argon and neon are used in “gas-filled” electric light bulbs and coloured “neon”
electrical signs. They are obtained from liquefied air.
Air pollutants
The air always contains small quantities of many gases. Such gases include hydrogen sulphide,
sulphur dioxide, as well as dust and other solid particles, especially in industrial areas. These
gases are given off during the combustion of coal, and the fuels resulting from coal.
SEPARATION OF AIR INTO ITS CONSTITUENT GASES
The air we breathe is necessary to keep us alive. It is also a chemical resource. Oxygen is used
in steel making, and nitrogen is used in making fertilizers. To use these gases in this way, they
must be separated from the atmospheric air. Air, as we studied in chapter 5, is a mixture of
different gases. The method used to separate its constituent gases is fractional distillation. The
gases have to be liquefied so that the mixture can be fractionally distilled.
The process of separating the air into its constituent gases is difficult. It cannot be done in the
laboratory. It is only done in industry. The chemical industry needs the gases from the air in
their pure form.
PREPARED BY MACDONALD RICHARD
The fractional distillation of air involves essentially two stages:
1. First, the air must be cooled until it turns into a liquid.
2. Then, the liquid air is allowed to warm up again. The various gases boil off at different
temperatures
Stage 1: Liquefaction of air
Air is filtered to remove any dust particles (purification).
The air is cooled to -180°C to remove the water vapour and carbon dioxide.
The air is then compressed to 100-150 atmospheres. As the compressed air gets very hot, it
has to be cooled.
The compressed cooled air is allowed to expand rapidly. The rapid expansion cools the air
to very low temperatures, and the liquid drops out. At -200°C, only helium and neon
remain as gases. The cold gases are used to cool the compressed air.
Stage 2: Fractional distillation of liquid air
The air is cooled and compressed to form liquid air. The liquid air is allowed to warm up.
Nitrogen boils off first because it has a low boiling point, -196°C. Argon follows by boiling at
-186°C and finally oxygen at -183°C
Figure above illustrates all the steps that take place during the process.
Fractional distillation of liquid air
PREPARED BY MACDONALD RICHARD
Combustion
The Concept of Combustion
Explain the concept of combustion
Combustion of a substance in oxygen or air is so common that it becomes almost a habit to use
the word "combustion" as if it referred to this kind of reaction alone. In real sense, it may be
applied to any chemical reaction accompanied by light and heat in which one or more of the
reactants are gaseous.
Many common substances burn in air. Substances such as coal, wood, kerosene, petrol, etc, burn
in air. Any substance that burns is called a combustible material. The air or oxygen that supports
the combustion is called a supporter of combustion. This is because we live in an atmosphere of
air that contains oxygen, which is a very reactive gas. The gas surrounds any burning material.
Oxygen is regarded as a supporter of combustion. However, it can sometimes combine
chemically with the burning substance to produce a new substance, as we shall see later.
Combustion of a substance involves its reaction with oxygen and the release of energy. These
reactions are exothermic and often produce a flame. An exothermic reaction is the one that is
accompanied by release of heat to the surrounding environment. Combustion in which a flame is
produced is described as burning. During burning energy is given out in the form of heat, light
and sound.
The Combustion of Different Substances in Air and Analyse the Products
Demonstrate the combustion of different substances in air and analyse the products
Many different substances burn in air to produce different products. Here are examples of
combustion of some common substances:
Sulphur
This is a yellow powder. When burnt in air, it gives misty fumes of sulphur dioxide gas.
Copper
When a piece of copper foil in a pair of tongs is held in a Bunsen flame, it becomes red-hot. On
cooling, a black layer of some substance is observed. This black substance is copper oxide. The
reaction occurs thus:
Magnesium
When one end of a piece of magnesium ribbon in tongs is placed in a Bunsen flame, it burns
with a dazzling flame leaving a white ash. This white ash is magnesium oxide.
PREPARED BY MACDONALD RICHARD
Hydrocarbons
Candle wax is a hydrocarbon. When it burns in air, the carbon and hydrogen of the wax react
with the oxygen of the air to give carbon dioxide and water vapour respectively.
These are substances containing carbon and hydrogen only. The burning of these organic
substances produces carbon dioxide and water vapour as the main products. If oxygen supply is
low, combustion is incomplete and carbon monoxide may be formed.
Coal
Coal is a solid fuel that will burn in air to give the following products:
The Application of Combustion in Real Life
Describe the application of combustion in real life
1. The combustion of a natural gas is an important source of energy for homes and industry.
Natural gas is mainly methane. Its complete combustion produces carbon dioxide and water
vapour.
Substances like methane, which undergo combustion readily and give out large amount of
energy, are known as fuels.
2. There are some reactions where fuels and other substances burn to produce a flame. These are
combustion reactions. There are also other combustion reactions (exothermic) where no flame is
evident. The most important of these is the crucial biochemical reaction that releases energy in
our body cells called cellular respiration.
Our bodies need energy to make possible the reactions that take place in our cells. These
reactions allow us to carry out our everyday activities. We need energy to stay alive. We get this
energy from food. During digestion, food is broken down into simpler substances. For example,
the carbohydrates in rice, potatoes and bread are broken down to form glucose. The combustion
of glucose with oxygen in the cells of our body provides energy.
The reaction is exothermic and is known as cellular respiration.
3. We combust fuels to heat homes and keep ourselves warm, cook our food, and even burn
wastes.
4. Combustion of fuels in automobile engines produces power (energy). This energy is supplied
to different parts of motor vehicles to make them move from one point to another or carry out
some crucial activities such as grinding, pumping, hauling etc. The operation of such machines
could be impossible without combustion of fuel that produces energy to make them work.
5. Combustion of fuel in different burners produces heat and light used for different purposes in
a chemistry laboratory.
PREPARED BY MACDONALD RICHARD
6. Extraction of metals: Moderately reactive metals such as zinc, iron and lead are roasted in a
special furnace (kiln) to form oxides. The resulting oxides are then reduced with carbon to get
the pure metal. This process of extracting a metal from its ore by heating is called smelting.
7. In metallurgical industry, combustion is used during welding. Welding is the process of
joining metals by melting the parts and then using a filler to form a joint. It can be done using
different energy sources, including a gas flame.
Fire fighting
Firefighting is the act of extinguishing destructive fires. A fire fighter fights these fires to
prevent destruction of life, property and the environment. Firefighting is a highly technical
profession that requires training and education in order to become proficient.
Types of Fires According to their Causes
Classify types of fires according to their causes
Before starting to fight the fire, it is important to know the size and type of the fire that you are
going to put off. The kind of firefighting material you are going to use will also depend on the
type of fire in question. Fires are classified based on the type of burning materials.
1. Class A fires
These are the fires in which the burning materials are ordinary combustible materials such as
paper, wood, cardboard, coal, rubber, clothing, furniture and most plastics. Water is the best
extinguisher for these fires. However, any other type of extinguisher, except carbon dioxide,
may be used.
2. Class B fires
These fires involve flammable liquids such as petrol, kerosene, oil, alcohol, ether, vanishes, etc.
For small fires, a fire blanket or sand may be used. If the fire is large, use foam, dry powder or
carbon dioxide extinguisher. Water should not be used on class B fires because the burning
material, being lighter than water, will just float and spread the fire further.
3. Class C fires
The burning material involves flammable gases e.g. hydrogen, acetylene, coal gas, butane,
methane, propane, etc. The best extinguishers to use in fighting against these fires are foam, dry
powder or carbon dioxide extinguishers. It is important to turn off the gas supply, and spray
water on the gas tank to cool it down.
4. Class D fires
The burning material is a metal. Alkali metals such as sodium or potassium may catch fire when
they come in contact with water and oxygen. At high temperatures, many metals react with
oxygen vigorously. Fires that involve burning metals should not be extinguished by water. This
is because the burning metal can react with water to give hydrogen (another potential fuel). The
appropriate extinguisher to use is foam or dry powder extinguisher.
5. Class E fires
These fires involve electrical equipment such as appliances, wiring, circuit breakers and outlets.
You may use carbon dioxide or dry powder extinguisher to put off these fires. Never use water
as it can conduct electricity and give an electric shock. Also remember to switch off power from
the mains.
6. Class F fires
The burning material is cooking oil or fat. A cooking oil fire in the kitchen can be extinguished
by covering the pan with a fire blanket or damp cloth. Foam, dry powder or carbon dioxide
extinguishers also work by cutting off the air supply to the fire. For large fires, wet chemical
extinguishers are recommended.
PREPARED BY MACDONALD RICHARD
Different Types of Fire Extinguishers used to Extinguish Different Types of Fire
Identify different types of fire extinguishers used to extinguish different types of fire
Before choosing the best fire extinguishers for fighting different types of fires it is crucial to
identify the type of burning materials first, and hence the type of fire such as:
Class A: Solids such as paper, wood, clothing, rubber, etc
Class B: Flammable liquids such as paraffin, petrol, oil, spirit, alcohol, etc.
Class C: Flammable gases such as propane, butane, methane, hydrogen, etc
Class D: Metals such as aluminium, magnesium, titanium, etc
Class E: Fires involving electrical equipment such as appliances, circuit breakers and outlets,
etc.
Types of fire extinguisher to use for each type of fire
Water extinguisher
This is the cheapest and most widely used fire extinguisher. It is used for class A fires. It is not
suitable for class B (liquid) fires, or where electricity is involved.
Foam extinguisher
This is more expensive than water extinguisher, but more versatile. It is used for classes A and B
fires. Foam spray extinguishers are not recommended for fires involving electricity, but are safer
than water if mistakenly sprayed onto live electrical apparatus.
Dry powder extinguisher
This is often termed as “multi-purpose” extinguisher, as it can be used on classes A, B and C
fires. It is the best for liquid fires (class B). It will also efficiently extinguish class C (gas) fires.
However, take care because it can be dangerous to extinguish a gas fire without first isolating
the gas supply. Special powders are available for class D fires.
When powder-type extinguishers are used indoors, the powder can obscure vision or damage
goods and machinery. It is also very messy.
Carbon dioxide extinguisher
Carbon dioxide is ideal for fires involving electrical apparatus (class E). It will also extinguish
class B (liquid) fires. However, the extinguisher has no post-fire security and the fire could re-
ignite.
Wet chemical extinguisher
This is a special extinguisher for class F fires. The extinguisher contains potassium salts. The
salts not only help to cool down the flames but also form a ‘saponification’ blanket that
effectively smothers the flames with thick, soapy foam.
Specialist powder extinguisher
This is a specialist fire extinguisher for use on class D fires (fires on combustible metals such as
sodium, potassium, magnesium, lithium, titanium, manganese and aluminium), especially in the
form of powder or turnings.
PREPARED BY MACDONALD RICHARD
The Components Needed to Start a Fire
State the components needed to start a fire
To extinguish fire, it is necessary to remove one or more of the three components of combustion.
Any fire needs a fuel, oxygen (air) and heat to keep it going. Remove any one of them and the
fire will go out. These components are as shown in the fire triangle below.
The fire triangle
A fire will continue or start to burn if these components are present:
(i) Fuel: This refers to any combustible material be it solid, liquid or gaseous material
provided it can catch fire and burn. You can stop fire by removing the combustible
material from the path of fire.
(ii) Oxygen (air): Oxygen supports combustion. A fuel will only burn if there is sufficient
supply of oxygen. You can extinguish fire by displacing, or taking away oxygen supply from the
fire or by blocking the gas supply to the fire.
(iii) Heat: The temperature should be at the kindling point of that fuel or above it. Every fuel
has its own kindling point. Below the kindling point, the fuel will not catch fire. You can put out
fire by lowering the temperature below the kindling point of a particular fuel. Water may be
used to cool down the fuel. The vapourization of water absorbs the heat; it cools the smoke, air,
walls, objects etc, which could be used as further fuel.
Fire Extinguishers According to the Chemicals they Contain
Classify fire extinguishers according to the chemicals they contain
Fire extinguishers are classified according to the type of chemicals they contain
1. Liquid carbon dioxide extinguisher
This extinguisher contains liquid carbon dioxide. The liquid is contained in a metal container.
When the safety pin is removed, carbon dioxide evaporates as solid "snow" (carbon dioxide
sublimes). The snow settles on the fire and suffocates it.
2. Soda-acid extinguisher
PREPARED BY MACDONALD RICHARD
This extinguisher has a metal case containing soda (aqueous sodium carbonate or sodium
hydrogen carbonate). In the metal case there is a glass bottle containing a concentrated acid
(sulphuric or hydrochloric acid). There is a knob attached to the top of a metal case. Hitting this
knob breaks the acid bottle thus bringing the acid and the soda into contact. The two react to
give carbon dioxide, e.g.
The gas forms bubbles with the solution, thereby forming foam which is forced out of a jet of
the case. The foam is directed to the fire where it covers the burning liquid, excluding all air
from reaching the fire.
Some extinguishers are made in such a way that turning them upside down brings the soda and
acid into contact and the reaction proceeds as stated above.
Soda-acid fire extinguishers
3. Foam extinguishers
This is different from the soda–acid type in that it contains sodium hydrogen carbonate in the
metal case, but instead of the concentrated acid, it contains aluminium sulphate and saponium in
the glass bottle. On mixing the three components, carbon dioxide gas is produced. The gas is
ejected out as foam. The foam here lasts longer than the foam in the soda–acid extinguisher. The
foam so produced also keeps air away from the burning material.
4. Dry chemical extinguisher
This extinguisher uses powdered sodium hydrogen carbonate and a nitrogen gas kept at high
pressure. When the gas cartridge is broken using the top cap, the carbon dioxide under pressure
propels the powder. The powder forms a layer over the burning material to keep air away.
Table bellow summarizes the types of fire extinguishers, indicating the chemicals they contain
and the classes of fire they are suitable or unsuitable for.
PREPARED BY MACDONALD RICHARD
Table: Types fire extinguishers and the chemical composition of their extinguishing agents
Type Chemical composition Suitable for Unsuitable for
of agent
APW (Air- Ordinary tap water Class A Class B, C, D and E (will
pressurized pressurized with air spread the flame and make
water) the fire bigger!)
Dry Fine sodium bicarbonate Class A, B, C - Class D- Aircraft and
chemical(DC) powder pressurized with and E electronics ( corrosive to
nitrogen metals such
aluminium)Note: -Though it
is safe to use indoors it can
obscure vision
CO2 Non-flammable carbon Class B, C and E Class A (leaves a flammable
dioxide gas under substance on the
extreme pressure extinguished material which
canre-ignite later )
Halon Bromochloro-difluoro- Class A and E Class B and C (least
methane suitable)
Foam Proteins and fluoro- Class A and B Class E
proteins
Wet Potassium acetate Class F Class E
chemical(WC)
ABC Mono-ammonium Class A, Class B Electronic equipment (leaves
phosphate with a and C a stick y residue that may be
nitrogen carrier damaging to electrical
appliances such as a
computer)
Specialist Powders of NaCl, Cu or Class D Class A, B, C, E and F
powder (SP) graphite under extreme
pressure
Precautions on using fire extinguishers
The following are some safety precautions you have to keep in mind when using fire
extinguishers:
1. Keep a reasonable distance from the fire as it may suddenly change direction.
2. Never use a portable extinguisher on people, instead use a fire blanket.
3. Do not test a portable extinguisher to see if it works. It may leak and later fail to work
during an emergency.
4. Do not return a used portable extinguisher to the wall. Make sure it is recharged first.
5. When a fire gets out of control, notify the nearest fire brigade.
Extinguishing Small Fires Using the Right Types of Fire Extinguishers
Extinguish small fires using the right types of fire extinguishers
Activity 1
Extinguish small fires using the right types of fire extinguishers
PREPARED BY MACDONALD RICHARD
Rusting
The Concept of Rusting
Explain the concept of rusting
Rusting is the name given to the oxidation of iron or steel in damp air. It is also called corrosion.
Rust is hydrated iron (III) oxide. It is a soft, crumbly solid and hence weakens the structure of
iron and steel. During rusting, iron reacts with oxygen to form brown iron (III) oxide
At the same time the iron (III) oxide reacts with water to form hydrated iron (III) oxide (or rust):
Note: The x in the equation indicates that the number of water molecules in the hydrated iron
(III) oxide can vary. So, both oxygen and water are needed to cause rusting of iron.
Rusting is a serious economic problem. Large sums of money are spent each year to replace
damaged iron and steel structures, or protecting structures from such damages. Rusting of
bridges, corrugated iron sheets on house roofs, containers, articles, etc. require an expenditure of
big sums of money as well as labour for replacement. Rust weakens structures such as car
bodies, iron railings, and ships’ hulls, and shortens their useful life. Preventing it can cost a lot
of money. All efforts must be made to stop iron or steel items from rusting. This can be achieved
if we know the conditions necessary for iron to rust.
The Conditions Necessary for Iron to Rust
Demonstrate the conditions necessary for iron to rust
When iron is left in contact with both water and oxygen (or air), it reacts to form hydrated iron
(III) oxide. Iron will not rust on exposure to dry air or air-free water (water that has been boiled
to expel all dissolved air) only. However, iron will easily and readily rust in water that has
dissolved air in it. In figure 6.8, only the iron nail that is in contact with both water and air rusts.
Therefore, rusting will only occur in the presence of both water and oxygen. If one of the two
conditions is excluded, in one way or another, rusting will not take place at all.
PREPARED BY MACDONALD RICHARD
Testing for conditions necessary for iron rusting
Findings
Nails in tube 1 will rust. Nails in tubes 2 and 3 will not rust.
Reasons
In tube 1, nails are in contact with both water and air (oxygen). In tube 2, the water has been
boiled to expel the dissolved air. In addition, any air above the water is prevented from
dissolving in boiled water by a layer of oil. So, the nails are completely shielded away from air.
Therefore, rusting is impossible. In tube 3, nails are in contact with air only. The moisture
present in air is absorbed by anhydrous calcium chloride. Any moisture that might have been
absorbed by the anhydrous calcium chloride is prevented from reaching the nails by a tuft of
cotton wool. The cotton wool also absorbs some moisture directly from the air. Therefore, tube 3
will always carry dry air (moisture-free air). Hence, no rusting of iron nails occurs.
This experiment demonstrates the fact that for iron to rust, both water and air (oxygen) must be
present. If one of these conditions is controlled, no rusting can take place.
Similarity between rusting and burning
Chemically, rusting and burning are similar processes in that they both require oxygen. Consider
the burning of magnesium to give magnesium oxide.
In this process, magnesium combines with the oxygen of the air to form magnesium oxide.
During rusting, iron combines with oxygen of the air in the presence of water to form brown
hydrated iron (III) oxide, "rust."
In addition, the two processes, burning and rusting, are exactly similar in that they both generate
heat. The only difference is in the time required for each of the two processes to take place.
During rusting heat is given out, but without being noticed because of its slower rate of
production. Burning produces noticeable heat and light.
The Different Methods of Preventing Iron from Rusting
Describe the different methods of preventing iron from rusting
We have learned that for iron to rust there must be direct contact between the iron and both
water and oxygen from the air. Therefore, in order to stop rusting we must protect iron from
PREPARED BY MACDONALD RICHARD
either water (moisture) or oxygen (air) or both. The following are some of the methods used to
prevent iron from rusting:
Painting
Painting the iron article creates a waterproof and airproof cover over the surface of the iron. This
method is widespread for objects ranging in size from ships and bridges to garden gates. Paints
that contain lead or zinc are mostly used. These paints are especially good for preventing
rusting. For example, "red lead" paints contain an oxide of lead,.
As oxygen and water cannot reach the iron, it does not rust. However, if the paint layer is
scratched off rusting may occur. So, regular repainting is necessary to keep this protection
intact.
Oiling and greasing
The oiling and/or greasing of the moving parts of machinery forms a protective film, preventing
rusting. Moving parts cannot be painted since the paint layer can be easily scratched off during
movement. Again, the treatment must be repeated to continue the protection.
Plastic coating
Steel is coated with plastic for use in garden chairs, refrigerators, bicycle baskets, dish racks, etc.
The plastic PVC (polyvinyl chloride), a trade name for polychloroethene, is often used for this
purpose. Plastic is cheap and can be made to look attractive.
Electroplating
Electroplating is the coating of one metal with a layer of another metal by means of electrolysis,
where the metal to be coated is the cathode and the coating metal the anode.
An iron or steel object can be electroplated with a layer of chromium or tin to protect against
rusting. A ‘tin can’ is made of steel coated on both sides with a fine layer of tin. Tin is used
because it is unreactive and non-toxic. However, if protective layer is broken, then the steel
beneath will begin to rust. So, proper handling of tin-plated items is needed.
Galvanizing
An iron object may be covered with a layer of zinc. This is called galvanizing. Even if the zinc
is scratched to expose the iron, the iron does not rust. This is because zinc is higher in the
reactivity series than iron. So, zinc reacts with water and oxygen in preference to iron.
The zinc layer can be applied by several different methods. These include electroplating or
dipping the object into molten zinc. When an iron or steel article is dipped into molten zinc and
then removed, it becomes coated with a thin layer of zinc. The zinc forms a protective coat over
the surface of iron. This process is used for dustbins, car bodies, barbed wires and motorway
crash barriers.
Sacrificial protection
This is a method of rust protection in which blocks of a metal more reactive than iron are
attached to the iron surface. Zinc and magnesium are more reactive than iron. When blocks of
zinc or magnesium are attached to the hull of a steel ship or oil rig, it corrodes in preference to
iron. This is called sacrificial protection because the zinc or magnesium is sacrificed to protect
the iron. When the blocks are nearly eaten away, they can be replaced by fresh blocks.
Underground gas and water pipes are connected by wire to blocks of magnesium to obtain the
same protection.
It is not necessary to cover the whole surface of a steel article with the more reactive metal for
sacrificial protection to work. A ship may have magnesium blocks riveted to its hull every few
metres to prevent rusting of the whole hull.
PREPARED BY MACDONALD RICHARD
Blocks of zinc (or magnesium) attached to the hull of a ship
Alloying
Alloys are mixtures of metals. For example, iron can be mixed with small quantities of much
less reactive metals to form an alloy called stainless steel. Stainless steel contains iron mixed
with chromium, nickel and manganese. Stainless steel does not rust. It also has a very attractive
appearance. It is used to make cutlery and kitchen equipment.
Use of silica gel
Silica is a common name for silicon dioxide (SiO2). Silica gel is a granular, vitreous, highly
porous form of silica made synthetically from sodium silicate. Despite its name, silica gel is a
solid. It is used as a desiccant, which absorbs moisture to prevent rusting of iron items or
articles. Most often, a small bag of silica gel is put inside bags or boxes used for storing or
carrying iron items to absorb any moisture that may cause rusting.
PREPARED BY MACDONALD RICHARD
You might also like
- The Composition and Importance of AirDocument50 pagesThe Composition and Importance of AirGenevieve Yong100% (1)
- Air and Combustion (1)Document38 pagesAir and Combustion (1)hassanpeter617No ratings yet
- IGCSE Chemistry - Oxygen, Hydrogen and Carbon DioxideDocument15 pagesIGCSE Chemistry - Oxygen, Hydrogen and Carbon DioxideChemistryKlipz75% (4)
- Composition of Air and Oxygen Percentage ExperimentsDocument18 pagesComposition of Air and Oxygen Percentage ExperimentsMaryam YousafNo ratings yet
- IGCSE Chemistry Oxygen Hydrogen and Carbon DioxideDocument15 pagesIGCSE Chemistry Oxygen Hydrogen and Carbon DioxideS M AkashNo ratings yet
- Air and Water ChemistryDocument24 pagesAir and Water ChemistryShaman Samuel GodfreyNo ratings yet
- Lesson 4 - Analysis of Flue GasDocument4 pagesLesson 4 - Analysis of Flue GasKamille NayraNo ratings yet
- Co AnalyserDocument6 pagesCo Analysersanjay sharmaNo ratings yet
- The Composition and Importance of AirDocument19 pagesThe Composition and Importance of AirummahputeriNo ratings yet
- Preparation and Properties of Oxygen ExperimentDocument10 pagesPreparation and Properties of Oxygen ExperimentGamolicaNo ratings yet
- William RamsayDocument5 pagesWilliam RamsayNicholas OwNo ratings yet
- Combustion Done - Solving NalangDocument2 pagesCombustion Done - Solving Nalangthercode sampNo ratings yet
- Air NotesDocument11 pagesAir NotesFatima AliNo ratings yet
- Chemical Changes Powerpoint - ChemistryDocument76 pagesChemical Changes Powerpoint - ChemistryghyaefuibNo ratings yet
- Industrial Gases: Nitrogen, Oxygen, Hydrogen ManufactureDocument13 pagesIndustrial Gases: Nitrogen, Oxygen, Hydrogen ManufactureBiain A SecasNo ratings yet
- Fuel L2 (1) ,,ATDocument30 pagesFuel L2 (1) ,,ATVishvas SinghhNo ratings yet
- Previous NextDocument7 pagesPrevious NextWajid HussainNo ratings yet
- Gaseous FuelsDocument8 pagesGaseous FuelsvaibhavNo ratings yet
- Lect. 2 (1)Document5 pagesLect. 2 (1)xa53dasNo ratings yet
- Athmosphere and Environment Research For O LevelsDocument12 pagesAthmosphere and Environment Research For O LevelsAsim HussainNo ratings yet
- The Atmosphere Lo2Document37 pagesThe Atmosphere Lo2Hailey CaruanaNo ratings yet
- Combustion Theory PPT OriginalDocument30 pagesCombustion Theory PPT Originalsameer betalNo ratings yet
- Flue Gas AnalysisDocument21 pagesFlue Gas AnalysisMuhammad AwaisNo ratings yet
- Mine GasesDocument17 pagesMine Gasessathish maramNo ratings yet
- Chapter 10-12Document51 pagesChapter 10-12jareef6969No ratings yet
- Volume Composition of Gases Present in Dry Air.: Nitrogen: Oxygen: Noble Gases: (Mainly) Carbon DioxideDocument28 pagesVolume Composition of Gases Present in Dry Air.: Nitrogen: Oxygen: Noble Gases: (Mainly) Carbon DioxideLee Jia YingNo ratings yet
- Chemistry FolioDocument43 pagesChemistry Folioharshini1010No ratings yet
- Combustion and Flue Gas Analysis: Excellence in MeasurementsDocument37 pagesCombustion and Flue Gas Analysis: Excellence in MeasurementsDaphne Cosi LealNo ratings yet
- Ombustion AND AFE Urnace Perations: C S F ODocument79 pagesOmbustion AND AFE Urnace Perations: C S F OTruth Seeker100% (1)
- Combustion TheoryDocument30 pagesCombustion TheoryYuvaraj KumarNo ratings yet
- AIR AND WATER COMPOSITIONDocument16 pagesAIR AND WATER COMPOSITIONtavongaNo ratings yet
- WINSEM2021-22 CHE2006 TH VL2021220501413 Reference Material I 11-03-2022 Module-5 CombustionDocument63 pagesWINSEM2021-22 CHE2006 TH VL2021220501413 Reference Material I 11-03-2022 Module-5 Combustionswastik vijayNo ratings yet
- Oxygen and oxides gases in airDocument15 pagesOxygen and oxides gases in airshaniakiwiNo ratings yet
- AIR NotesDocument5 pagesAIR NotesjpkaomeNo ratings yet
- Unit 42: Heat Transfer and Combustion: Unit Code: K/601/1443 QCF Level: 5 Credit Value: 15Document20 pagesUnit 42: Heat Transfer and Combustion: Unit Code: K/601/1443 QCF Level: 5 Credit Value: 15david19890109No ratings yet
- Chemistry Project (1) (Mohamed Part)Document6 pagesChemistry Project (1) (Mohamed Part)mohamed amirNo ratings yet
- Diesel Coal Air Fuel RatioDocument2 pagesDiesel Coal Air Fuel RatioEben Ronitua SitompulNo ratings yet
- Gaseous FuelDocument20 pagesGaseous FuelCaguioa Mark Anthony G.100% (3)
- Lecture Notes 5A - Single-Phase System Exercise 1Document26 pagesLecture Notes 5A - Single-Phase System Exercise 1TaanzNo ratings yet
- Q1. Write Briefly On Composition of Atmosphere Ans.: It Is Caused by Burning Fossil Fuels, Like Coal and PetroleumDocument6 pagesQ1. Write Briefly On Composition of Atmosphere Ans.: It Is Caused by Burning Fossil Fuels, Like Coal and PetroleumRonnith NandyNo ratings yet
- Kiln Emissions - More Than Just Hot Air: Authoer:WCEO-AdminDocument8 pagesKiln Emissions - More Than Just Hot Air: Authoer:WCEO-AdminGilberto PérezNo ratings yet
- Combustion of FuelDocument16 pagesCombustion of FuelRizuanul Arefin EmonNo ratings yet
- Prepared By: Lee Hock TiangDocument27 pagesPrepared By: Lee Hock TiangNick LeeNo ratings yet
- CO2 ProductionDocument111 pagesCO2 ProductionGhufran SaeedNo ratings yet
- Air and Water: Measuring The Percentage of Oxygen in AirDocument3 pagesAir and Water: Measuring The Percentage of Oxygen in AirJoseph LimNo ratings yet
- Nptel 1 PDFDocument9 pagesNptel 1 PDFShubham KumarNo ratings yet
- Topic.10 Chemistry of Our EnviromentDocument12 pagesTopic.10 Chemistry of Our EnviromentJoyce AmirNo ratings yet
- Assignment of CombustionDocument9 pagesAssignment of CombustionAfiq de WinnerNo ratings yet
- Combustion of AlkanesDocument7 pagesCombustion of AlkanesA-ar FebreNo ratings yet
- Importance of Air CompositionDocument38 pagesImportance of Air CompositionNoorizan Mohd EsaNo ratings yet
- ICSE Selina Solution For Class 9 Chemistry Chapter 8 Exercise QuestionsDocument16 pagesICSE Selina Solution For Class 9 Chemistry Chapter 8 Exercise QuestionsYash KapoorNo ratings yet
- Thermodynamic Principles of Combustion ProcessesDocument12 pagesThermodynamic Principles of Combustion ProcessesArjun LutchumunNo ratings yet
- Manufacture Nitric AcidDocument9 pagesManufacture Nitric AcidDjayustinus Heri HermawanNo ratings yet
- Combustion of FuelDocument16 pagesCombustion of Fuelpragna patelNo ratings yet
- Revision NotesDocument5 pagesRevision Notessophie hareNo ratings yet
- 2021 Asoe Jso Exam AnswersDocument26 pages2021 Asoe Jso Exam AnswersLei ZhangNo ratings yet
- Sample 1845Document16 pagesSample 1845Thala AjithNo ratings yet
- A System of Instruction in the Practical Use of the Blowpipe: Being A Graduated Course Of Analysis For The Use Of Students And All Those Engaged In The Examination Of Metallic CombinationsFrom EverandA System of Instruction in the Practical Use of the Blowpipe: Being A Graduated Course Of Analysis For The Use Of Students And All Those Engaged In The Examination Of Metallic CombinationsNo ratings yet
- Topic 3 Major Features of The Earth's SurfaceDocument7 pagesTopic 3 Major Features of The Earth's SurfaceTrump DonaldNo ratings yet
- Topic 4 Scientific Procedures.Document8 pagesTopic 4 Scientific Procedures.Trump DonaldNo ratings yet
- Topic 1 Introduction To ChemistryDocument6 pagesTopic 1 Introduction To ChemistryTrump DonaldNo ratings yet
- Topic 5 MatterDocument20 pagesTopic 5 MatterTrump DonaldNo ratings yet
- Topic 1 Introduction To ChemistryDocument6 pagesTopic 1 Introduction To ChemistryTrump DonaldNo ratings yet
- Topic 5 Map WorkDocument6 pagesTopic 5 Map WorkTrump DonaldNo ratings yet
- Topic 4 WeatherDocument15 pagesTopic 4 WeatherTrump DonaldNo ratings yet
- Topic 3 Heat Sources and FlamesDocument7 pagesTopic 3 Heat Sources and FlamesTrump DonaldNo ratings yet
- Topic 2 The Solar SystemDocument12 pagesTopic 2 The Solar SystemTrump DonaldNo ratings yet
- Lesson Iv Esau and Jacob (Israel)Document2 pagesLesson Iv Esau and Jacob (Israel)Trump DonaldNo ratings yet
- Topic 1 Concept of GeographyDocument2 pagesTopic 1 Concept of GeographyTrump DonaldNo ratings yet
- Lesson Xii Book of ActsDocument4 pagesLesson Xii Book of ActsTrump DonaldNo ratings yet
- Lesson Xi Gospel of MatthewDocument8 pagesLesson Xi Gospel of MatthewTrump DonaldNo ratings yet
- Lesson X Israel Under The Leadership of SamuelDocument1 pageLesson X Israel Under The Leadership of SamuelTrump DonaldNo ratings yet
- Lesson Vii The Census of The Israelites at Sinai.Document2 pagesLesson Vii The Census of The Israelites at Sinai.Trump DonaldNo ratings yet
- Joshua Leads Israel to Victory in the Promised LandDocument2 pagesJoshua Leads Israel to Victory in the Promised LandTrump DonaldNo ratings yet
- Lesson Ix Israel Under The Leadership of JudgesDocument5 pagesLesson Ix Israel Under The Leadership of JudgesTrump DonaldNo ratings yet
- LN17 PDFDocument4 pagesLN17 PDFTrump DonaldNo ratings yet
- Lesson Iii The Great FloodDocument3 pagesLesson Iii The Great FloodTrump DonaldNo ratings yet
- Introducing The BibleDocument8 pagesIntroducing The BibleTrump DonaldNo ratings yet
- Lesson I CreationDocument3 pagesLesson I CreationTrump DonaldNo ratings yet
- Lesson Ii The Fall of Man & Its ConcequencesDocument3 pagesLesson Ii The Fall of Man & Its ConcequencesTrump DonaldNo ratings yet
- Fee Structure For Certificate & Diploma Programs 2019-2020 Including Schedule of PaymentDocument2 pagesFee Structure For Certificate & Diploma Programs 2019-2020 Including Schedule of PaymentTrump DonaldNo ratings yet
- Topic 2: Fractions: Describe A FractionDocument6 pagesTopic 2: Fractions: Describe A FractionTrump DonaldNo ratings yet
- Zanzibar Library Services Field ReportDocument24 pagesZanzibar Library Services Field ReportTrump Donald100% (1)
- Topic 1 NumbersDocument16 pagesTopic 1 NumbersTrump DonaldNo ratings yet
- Field Report PDFDocument30 pagesField Report PDFMaann KatJacobNo ratings yet
- Challenges and Solutions for Diverse EntrepreneursDocument23 pagesChallenges and Solutions for Diverse EntrepreneursWin BoNo ratings yet
- Spyhuman: Select Type Mobile No. Duration Date & TimeDocument1 pageSpyhuman: Select Type Mobile No. Duration Date & TimeTrump DonaldNo ratings yet
- Excerpts From TNO Purple BookDocument6 pagesExcerpts From TNO Purple BookraritylimNo ratings yet
- C7012A, C, E, F, G Solid State Purple Peeper® Ultraviolet Flame DetectorsDocument20 pagesC7012A, C, E, F, G Solid State Purple Peeper® Ultraviolet Flame DetectorsMauricio GuanellaNo ratings yet
- Fire Safety Journal: Filip Van Den Schoor, Prankul Middha, Eric Van Den BulckDocument11 pagesFire Safety Journal: Filip Van Den Schoor, Prankul Middha, Eric Van Den BulckhsebillalNo ratings yet
- Lfe 50 N 7783 en 09052007Document14 pagesLfe 50 N 7783 en 09052007Thiago FernandesNo ratings yet
- At2000st RepairDocument56 pagesAt2000st RepairJohn Smith100% (1)
- SpektrofotometerDocument8 pagesSpektrofotometerTitik FadilahNo ratings yet
- Westbrook Dryer 1981 PDFDocument13 pagesWestbrook Dryer 1981 PDFVinícius Martins FreireNo ratings yet
- Lava Heat Italia - Ember Mini Patio Heater - Owners ManualDocument18 pagesLava Heat Italia - Ember Mini Patio Heater - Owners ManuallavaheatitaliaNo ratings yet
- Rogue Trader - Converted Dark Heresy Psychic Powers PDFDocument13 pagesRogue Trader - Converted Dark Heresy Psychic Powers PDFWhauknosrongNo ratings yet
- Santin Burners Web Catalogue 2018 PDFDocument78 pagesSantin Burners Web Catalogue 2018 PDFAlvaro RJNo ratings yet
- Home Smoke Alarm Tests in Manufactured HomesDocument2 pagesHome Smoke Alarm Tests in Manufactured HomesvytoNo ratings yet
- Weishaupt Monarch Oil Burners PDFDocument32 pagesWeishaupt Monarch Oil Burners PDFluisgabrielperez100% (2)
- CHE 029 - Exercise No 1Document3 pagesCHE 029 - Exercise No 1ronduexNo ratings yet
- C7061 Datasheet PDFDocument12 pagesC7061 Datasheet PDFMaxluthorNo ratings yet
- Eberspacher Repair AIRTRONIC D2-D4Document32 pagesEberspacher Repair AIRTRONIC D2-D4Daniel OprescuNo ratings yet
- Combustion in Si EnginesDocument36 pagesCombustion in Si Enginesrgopi_83No ratings yet
- Austin Marr - Lab 2 Flame Test InquiryDocument3 pagesAustin Marr - Lab 2 Flame Test Inquiryapi-427302061No ratings yet
- Nandha Engineering College (Autonomous) : 17mex12 - Internal Combustion EnginesDocument36 pagesNandha Engineering College (Autonomous) : 17mex12 - Internal Combustion EnginesSugumar MuthusamyNo ratings yet
- Research 1: Quarter 1 - Module 11: Analyzing and Interpreting DataDocument29 pagesResearch 1: Quarter 1 - Module 11: Analyzing and Interpreting DataEd Dioso100% (1)
- Cairo North ManualDocument982 pagesCairo North Manualsmart_eng2009100% (2)
- Dokumen - Tips - Army TM 9 6115 729 24 Air Force To 35c2 3 519 2 Marine PDFDocument1,013 pagesDokumen - Tips - Army TM 9 6115 729 24 Air Force To 35c2 3 519 2 Marine PDFABDUL QADIRNo ratings yet
- Eks 1Document6 pagesEks 1Medline TahaNo ratings yet
- Ultra Low Nox Burners PDFDocument6 pagesUltra Low Nox Burners PDFSteve WanNo ratings yet
- CHE Facts - Industrial Gas Burners - July 2016Document1 pageCHE Facts - Industrial Gas Burners - July 2016John UrdanetaNo ratings yet
- RLS 70-100-130 - eDocument28 pagesRLS 70-100-130 - eJosep Alexander Gutierrez ReyesNo ratings yet
- Burners - ACMCDocument19 pagesBurners - ACMCMuhammad ZaghloulNo ratings yet
- Lesson Plan in Science IVDocument4 pagesLesson Plan in Science IVPrecilla Ugarte Halago100% (10)
- U1 L8 Incomplete Combustion PDFDocument7 pagesU1 L8 Incomplete Combustion PDFHussein SayedNo ratings yet
- Properties and Transformation of MatterDocument3 pagesProperties and Transformation of Mattermari_kkkk100% (1)
- DURAG - Fibre Optic SystemDocument36 pagesDURAG - Fibre Optic SystemnpetruselliNo ratings yet