Professional Documents
Culture Documents
Complications Associated With Injectable Soft-Tissue Fillers
Uploaded by
Armin ArceoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Complications Associated With Injectable Soft-Tissue Fillers
Uploaded by
Armin ArceoCopyright:
Available Formats
ORIGINAL ARTICLE
Complications Associated With Injectable
Soft-Tissue Fillers
A 5-Year Retrospective Review
Steven M. Daines, MD; Edwin F. Williams III, MD
Importance: Even when administered by experienced 1, 2007, and December 31, 2011, and identified as hav-
hands, injectable soft-tissue fillers can cause various un- ing a treatment-related complication.
intended reactions, ranging from minor and self-limited
responses to severe complications requiring prompt treat- Results: A total of 2089 injectable soft-tissue filler treat-
ment and close follow-up. ments were performed during the study period, includ-
ing 1047 with hyaluronic acid, 811 with poly-L-lactic acid,
Objectives: To review the complications associated with and 231 with calcium hydroxylapatite. Fourteen com-
injectable soft-tissue filler treatments administered in the plications were identified. The most common complica-
Williams Rejuva Center during a 5-year period and to tion was nodule or granuloma formation. Treatment with
discuss their management. calcium hydroxylapatite had the highest complication rate.
Design and Setting: Retrospective medical record re- Conclusions and Relevance: Complications are rare
view in a private practice setting. following treatment with injectable soft-tissue fillers. Nev-
ertheless, it is important to be aware of the spectrum of
Participants: Patients receiving injectable soft-tissue fill- potential adverse sequelae and to be comfortable with their
ers and having a treatment-related complication. proper management.
Interventions: Injectable soft-tissue filler treatments. Level of Evidence: 4.
Main Outcome Measures: A retrospective medical rec- JAMA Facial Plast Surg. 2013;15(3):226-231.
ord review was conducted of patients undergoing treat- Published online March 28, 2013.
ment with injectable soft-tissue fillers between January doi:10.1001/jamafacial.2013.798
V
OLUME ENHANCEMENT HAS istered by non–core physicians and other
long been recognized as a medical practitioners.
means to achieve facial re- Even when administered by experi-
juvenation. Various soft- enced hands, injectable fillers can cause
tissue fillers have gained various unintended reactions, ranging from
popularity among aesthetic surgeons and minor and self-limited responses to se-
patients alike for their rapid and predict- vere complications requiring prompt treat-
able results, relative ease of delivery, and ment and close follow-up. The aesthetic
favorable safety profiles. Over the years, surgeon should not only have a firm un-
as patients have developed an almost in- derstanding of the potential complica-
satiable appetite for soft-tissue augmen- tions caused by injectable fillers but also
tation with injectable fillers, the pool of
know when to intervene and be confi-
providers has expanded from aesthetic sur-
geons and dermatologists to include health dent in managing the entire spectrum of
care practitioners with little or no formal adverse sequelae.
training in aesthetic medicine. In this study, we review 5 years of in-
Author Affiliations: Dr Daines A recent study 1 conducted by the jectable filler treatments administered in Author Aff
is in private practice at Daines American Society of Plastic Surgeons re- the Williams Rejuva Center to determine is in private
Plastic Surgery in Newport vealed a 190% increase in the number of the number and types of complications that Plastic Surg
Beach, California. Dr Williams occurred and how they were managed. We Beach, Cali
is in private practice at the
soft-tissue filler treatments performed by is in private
Williams Center Plastic Surgery plastic surgeons between 2000 and 2011. also searched the literature to provide treat- Williams C
Specialists in Latham, This astounding number does not reflect ment options for the more commonly ob- Specialists i
New York. the explosion in filler treatments admin- served adverse reactions. New York.
JAMA FACIAL PLAST SURG/ VOL 15 (NO. 3), MAY/JUNE 2013 WWW.JAMAFACIAL.COM
226
©2013 American Medical Association. All rights reserved.
Downloaded From: http://archfaci.jamanetwork.com/ by a New York University User on 05/17/2015
Table. Breakdown of Injectable Soft-Tissue Filler Types and Their Associated Complications
No. (%) of Complications
Filler Type (Total No.
of Treatments) Cellulitis Nodule or Granuloma Formation Skin Necrosis Other Total
Hyaluronic acid (n = 1047) 0 1 (0.1) 0 1 (0.1) 2 (0.2)
Poly-L-lactic acid (n = 811) 0 5 (0.6) 0 1 (0.1) 6 (0.7)
Calcium hydroxylapatite (n = 231) 4 (1.7) 1 (0.4) 1 (0.4) 0 6 (2.6)
All fillers (N = 2089) 4 (0.2) 7 (0.3) 1 (0.05) 2 (0.1) 14 (0.7)
METHODS other 2 cases of nodules were palpable but not visible,
although the nodules were associated with a general-
We queried the billing database to determine the total number ized induration of the midface in one of these patients.
of soft-tissue injectable filler treatments administered in the Wil- Hyaluronic acid injections were the least likely to re-
liams Rejuva Center between January 1, 2007, and December sult in a complication in this series. One patient devel-
31, 2011. Neurotoxin injections were not included in our analy- oped an inflammatory granuloma after treatment of her
sis. The treatments were further categorized as hyaluronic acid, lips with hyaluronic acid. A second patient experienced
poly-L-lactic acid, and calcium hydroxylapatite. In practice, we the Tyndall phenomenon after injection of hyaluronic acid
routinely see all patients treated with hyaluronic acid or cal- to treat a tear trough deformity.
cium hydroxylapatite 2 weeks after treatment for reassess-
ment, whereas patients undergoing treatment with poly-L-
lactic acid are seen approximately 8 to 12 weeks after they are COMMENT
treated. Any adverse reactions noted at a follow-up visit or re-
ported at any other time by the patient are documented in their Injectable soft-tissue fillers offer patients noticeable and
clinic medical record, as well as in a monthly quality assur-
ance report. To determine the total number of complications
immediate results, without the downtime associated with
associated with injectable soft-tissue fillers during this inter- traditional facial rejuvenation surgery. Whereas mild pain,
val, we reviewed the medical records of all the patients iden- swelling, and bruising at the injection site are common
tified in the quality assurance reports as having a filler-related and expected consequences of these treatments, true com-
complication. plications arise less commonly.
RESULTS VASCULAR OCCLUSION AND SKIN NECROSIS
Between January 1, 2007, and December 31, 2011, a total Although rare, skin necrosis is a potentially devastating
of 2089 soft-tissue filler injections were performed in the complication of treatment with injectable fillers. Necro-
office for volume augmentation. These injectable treat- sis is caused by vascular compromise resulting from ar-
ments consisted of the following: 1047 hyaluronic acid, terial or venous obstruction.2 The occlusion of blood flow
811 poly-L-lactic acid, and 231 calcium hydroxylapa- can be due to trauma to the vessel wall, inadvertent in-
tite. Complications were defined as adverse events not travascular injection of the product, or a direct pressure
considered expected sequelae of the injection itself (ie, effect of the filling agent on the vessel causing obstruc-
swelling, bruising, pain, and others). During the 5-year tion of the vessel lumen.3,4 Furthermore, injection-
period reviewed, 14 complications were identified: 2 in- related edema can compromise blood flow by contrib-
volved treatment with hyaluronic acid, 6 with poly-L- uting an external force on the vessel wall.
lactic acid, and 6 with calcium hydroxylapatite. The filler Arterial compromise is typically heralded by imme-
type and number of complications are summarized in the diate-onset blanching and severe pain, whereas venous
Table. obstruction frequently manifests with a delayed reticu-
Calcium hydroxylapatite was the filler most com- lated, violaceous appearance.2 If untreated, injectable-
monly associated with complications in this series. Three related vascular compromise (IRVC) will progress to a
patients developed cellulitis at the treatment site, one of partial-thickness or full-thickness injury to the skin, lead-
whom developed cellulitis on 2 separate occasions fol- ing to necrosis, skin slough, and scarring.
lowing treatments administered 7 months apart. One pa- Various algorithms have been described to manage
tient developed a submucosal nodule visible beneath the IRVC, although none are supported by more than anec-
buccal mucosa, and another patient experienced partial- dotal evidence.2-8 If IRVC is recognized during the treat-
thickness necrosis at the alar-facial junction following in- ment session, the injection should immediately be
jection of calcium hydroxylapatite into her melolabial stopped, and an attempt should be made to aspirate the
folds. product. Measures should then be implemented to im-
Patients treated in the office with poly-L-lactic acid prove blood flow and dissipate the agent. These include
experienced 6 total complications, including 5 cases of aggressive massage, warm compresses, and the applica-
subcutaneous nodules and 1 case of perioral dermatitis. tion of nitroglycerin to the affected area.6-8 When the cul-
In a patient with a history of sarcoidosis, the nodules were prit filler is hyaluronic acid, hyaluronidase can also be
visible at rest, while in 2 other patients the nodules were administered to digest the offending product.9 A course
barely perceptible with extreme facial animation. The of aspirin has also been advocated in these circum-
JAMA FACIAL PLAST SURG/ VOL 15 (NO. 3), MAY/JUNE 2013 WWW.JAMAFACIAL.COM
227
©2013 American Medical Association. All rights reserved.
Downloaded From: http://archfaci.jamanetwork.com/ by a New York University User on 05/17/2015
ORIGINAL ARTICLE
Complications Associated With Injectable
Soft-Tissue Fillers
A 5-Year Retrospective Review
Steven M. Daines, MD; Edwin F. Williams III, MD
Importance: Even when administered by experienced 1, 2007, and December 31, 2011, and identified as hav-
hands, injectable soft-tissue fillers can cause various un- ing a treatment-related complication.
intended reactions, ranging from minor and self-limited
responses to severe complications requiring prompt treat- Results: A total of 2089 injectable soft-tissue filler treat-
ment and close follow-up. ments were performed during the study period, includ-
ing 1047 with hyaluronic acid, 811 with poly-L-lactic acid,
Objectives: To review the complications associated with and 231 with calcium hydroxylapatite. Fourteen com-
injectable soft-tissue filler treatments administered in the plications were identified. The most common complica-
Williams Rejuva Center during a 5-year period and to tion was nodule or granuloma formation. Treatment with
discuss their management. calcium hydroxylapatite had the highest complication rate.
Design and Setting: Retrospective medical record re- Conclusions and Relevance: Complications are rare
view in a private practice setting. following treatment with injectable soft-tissue fillers. Nev-
ertheless, it is important to be aware of the spectrum of
Participants: Patients receiving injectable soft-tissue fill- potential adverse sequelae and to be comfortable with their
ers and having a treatment-related complication. proper management.
Interventions: Injectable soft-tissue filler treatments. Level of Evidence: 4.
Main Outcome Measures: A retrospective medical rec- JAMA Facial Plast Surg. 2013;15(3):226-231.
ord review was conducted of patients undergoing treat- Published online March 28, 2013.
ment with injectable soft-tissue fillers between January doi:10.1001/jamafacial.2013.798
V
OLUME ENHANCEMENT HAS istered by non–core physicians and other
long been recognized as a medical practitioners.
means to achieve facial re- Even when administered by experi-
juvenation. Various soft- enced hands, injectable fillers can cause
tissue fillers have gained various unintended reactions, ranging from
popularity among aesthetic surgeons and minor and self-limited responses to se-
patients alike for their rapid and predict- vere complications requiring prompt treat-
able results, relative ease of delivery, and ment and close follow-up. The aesthetic
favorable safety profiles. Over the years, surgeon should not only have a firm un-
as patients have developed an almost in- derstanding of the potential complica-
satiable appetite for soft-tissue augmen- tions caused by injectable fillers but also
tation with injectable fillers, the pool of
know when to intervene and be confi-
providers has expanded from aesthetic sur-
geons and dermatologists to include health dent in managing the entire spectrum of
care practitioners with little or no formal adverse sequelae.
training in aesthetic medicine. In this study, we review 5 years of in-
Author Affiliations: Dr Daines A recent study 1 conducted by the jectable filler treatments administered in Author Aff
is in private practice at Daines American Society of Plastic Surgeons re- the Williams Rejuva Center to determine is in private
Plastic Surgery in Newport vealed a 190% increase in the number of the number and types of complications that Plastic Surg
Beach, California. Dr Williams occurred and how they were managed. We Beach, Cali
is in private practice at the
soft-tissue filler treatments performed by is in private
Williams Center Plastic Surgery plastic surgeons between 2000 and 2011. also searched the literature to provide treat- Williams C
Specialists in Latham, This astounding number does not reflect ment options for the more commonly ob- Specialists i
New York. the explosion in filler treatments admin- served adverse reactions. New York.
JAMA FACIAL PLAST SURG/ VOL 15 (NO. 3), MAY/JUNE 2013 WWW.JAMAFACIAL.COM
226
©2013 American Medical Association. All rights reserved.
Downloaded From: http://archfaci.jamanetwork.com/ by a New York University User on 05/17/2015
derwent treatment of the prejowl region with a calcium
hydroxylapatite filler. One day later, she developed an
area of erythema and swelling along the chin and lower
cheek that was indurated and warm to the touch. She was
diagnosed as having cellulitis and oral antibiotics were
started. The affected area blistered and desquamated and
was then treated with topical emollients until healing was
complete. The cellulitis completely resolved, with no long-
term sequelae.
One patient developed 2 distinct episodes of celluli-
tis of the cheek after treatment sessions with calcium hy-
droxylapatite separated by 7 months. The second epi-
sode was more fulminant, with extensive erythema and
numerous small pustules. Given the recrudescence of her
infection during such a lengthy interval, the specter of
bacterial biofilm formation was considered. Fortu-
nately, both infections resolved with conservative medi-
cal management.
NODULES AND GRANULOMAS
Subcutaneous nodules are a known complication of in-
jectable fillers. Fibrotic nodules may arise from stimu-
latory products such as poly-L-lactic acid and calcium
hydroxylapatite. They tend to be painless, appear weeks
to months following treatment, and can last for years. In
most cases, they are palpable but cannot be seen. Al-
though the nodules usually remain localized to the site
of treatment, a degree of migration can also occur. In ex-
treme cases, they can become visible and are a source of Figure 3. Diffuse visible subcutaneous nodules following treatment with
poly-L-lactic acid.
significant anxiety for the affected patient.14
Subcutaneous nodules have been reported in 0% to
40% of patients receiving poly-L-lactic acid.15,16 Local- Although the prescribing information provided by the
ized accumulations of product are the most common cause manufacturer of injectable poly-L-lactic acid recom-
of nodules in patients receiving calcium hydroxylapa- mends reconstitution of 1 vial of product with 5 mL of
tite or hyaluronic acid, whereas fibrotic nodules are usu- sterile water,20 we prefer an off-label 8:1 dilution. In ad-
ally seen with stimulatory products, such as poly-L- dition, we reconstitute the product 48 hours before in-
lactic acid.17 Furthermore, certain anatomical sites are jection and use a laboratory vortex to agitate the mix-
known to present a higher risk for nodule formation, par- ture before its transfer for injection. We believe that these
ticularly the lower eyelid. Overall, a decrease in nodule simple measures in conjunction with meticulous injec-
formation may be seen with subdermal injection, postin- tion technique are responsible for our very low rate of
jection massage, a higher reconstitution volume, and a nodule formation (0.6%).
longer latency between reconstitution and injection.18,19 Nodules can also represent an infectious, immune, or
In this series, one instance occurred of an accumula- inflammatory process resulting in granuloma forma-
tion of calcium hydroxylapatite visible beneath the buc- tion.21 A granuloma can be differentiated from a fibrotic
cal mucosa due to an inadvertent depot delivery of filler nodule by its later onset, tenderness, swelling, possible
in this region. Five cases of apparent fibrotic nodule for- erythema, and occasional suppuration. Proposed causes
mation also occurred in patients receiving poly-L-lactic include biofilms, protein impurities, and irregularities of
acid. One patient, a 66-year-old woman with a history surface microspheres.13 Histologically, these nodules show
of sarcoidosis, underwent a series of 3 treatments dur- a foreign-body response with epithelioid granulomas and
ing 5 months to the midface, melolabial folds, and pre- an inflammatory infiltrate surrounding the inert prod-
jowl regions. Six months after her final treatment, she uct.22 With poly-L-lactic acid, it is possible that pro-
was noted to have diffuse beadlike nodules throughout cesses resulting in localized inflammation, such as sun-
her treatment areas (Figure 3). Unlike the other 4 pa- burn, trauma, or treatment of the skin with a laser, could
tients in the series who developed fibrotic nodules that stimulate inflammatory nodule formation.
were only apparent on palpation, hers were visible at rest. Hyaluronic acid fillers can also form intradermal granu-
Although the bumps were not painful and demon- lomas that are characterized by abundant multinucle-
strated no other inflammatory characteristics, we sur- ated giant cells surrounding the basophilic product.23 Hy-
mised that the sarcoidosis may have had a role in her de- aluronidase injections are the initial treatment of choice.9
veloping such an extensive distribution of nodules. She One patient in this series developed an inflammatory
was followed expectantly and showed no further nod- granuloma after undergoing augmentation of the upper
ule progression. and lower lips with hyaluronic acid filler. Approxi-
JAMA FACIAL PLAST SURG/ VOL 15 (NO. 3), MAY/JUNE 2013 WWW.JAMAFACIAL.COM
229
©2013 American Medical Association. All rights reserved.
Downloaded From: http://archfaci.jamanetwork.com/ by a New York University User on 05/17/2015
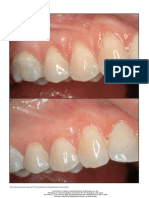
Figure 4. Inflammatory nodule of the left lower lip after treatment with
hyaluronic acid filler.
mately 2 months after injection, she noted painful swell-
ing of the lower lip and was found to have nonfluctuant
swelling and induration confined to the left side of the
lower lip (Figure 4). She was treated with a course of
antibiotics and a tapering course of oral corticosteroids,
and the swelling completely resolved during the next few
weeks. Because of the delayed presentation, this was
thought to be an inflammatory granuloma, with biofilm
formation a possible culprit.
During the 5-year study period, a patient with chronic
inflammatory granulomas was managed who had under- Figure 5. Inflammatory nodules in the prejowl region due to silicone
gone perioral silicone injections in another city. She was injection.
seen in the office intermittently reporting pain, redness,
and swelling around her mouth and chin (Figure 5) and
was treated on each occasion with oral and intralesional
corticosteroids. This chronic waxing-and-waning inflam- panding population of patients seeking injectable soft-
matory response is a hallmark of granulomas resulting tissue fillers may be vulnerable to complications that go
from soft-tissue injection of silicone. Because of the po- underrecognized and undertreated due to physician shop-
tentially devastating consequences associated with in- ping, limited follow-up care, and the lack of training
jectable silicone, we strongly recommend against its use among many practitioners performing filler treatments.
for volume augmentation. Infection, fibrotic nodules, granuloma formation, and vas-
cular compromise are possible sequelae that must be rec-
CONCLUSIONS ognized and managed properly to prevent potentially per-
manent disfigurement.
A significant shortcoming of this study was the retro-
spective nature of its design. Furthermore, despite a policy
to see all patients treated with injectable soft-tissue fill- Accepted for Publication: November 18, 2012.
ers for scheduled follow-up visits and to document all Published Online: March 28, 2013. doi:10.1001/jamafacial
complications in the quality assurance reports, it is pos- .2013.798
sible that we were unable to capture every adverse event Correspondence: Steven M. Daines, MD, Daines Plastic
that occurred during the study period. Some patients may Surgery, 180 Newport Center Dr, Ste 158, Newport Beach,
have failed to recognize an adverse event, did not return CA 92660 (dainesmd@gmail.com).
for their follow-up visits, or sought care for a complica- Author Contributions: Dr Daines had full access to all
tion with another physician. the data in the study and takes responsibility for the in-
Volume loss, an integral component of the facial ag- tegrity of the data and the accuracy of the data analysis.
ing process, is responsible for some of the telltale signs Study concept and design: Daines and Williams. Acquisi-
of senescence. Therefore, volume restoration, alone or tion of data: Daines. Analysis and interpretation of data:
in conjunction with other procedures, has become an im- Daines. Drafting of the manuscript: Daines. Critical revi-
portant focus of most facial rejuvenation strategies. Soft- sion of the manuscript for important intellectual content:
tissue injectable fillers have been and will continue to be Daines and Williams. Statistical analysis: Daines. Admin-
popular because of their minimal downtime, favorable istrative, technical, and material support: Daines. Study su-
safety profile, and rapid and reproducible results. Nev- pervision: Williams.
ertheless, injectables are not without risk. Today’s ex- Conflict of Interest Disclosures: None reported.
JAMA FACIAL PLAST SURG/ VOL 15 (NO. 3), MAY/JUNE 2013 WWW.JAMAFACIAL.COM
230
©2013 American Medical Association. All rights reserved.
Downloaded From: http://archfaci.jamanetwork.com/ by a New York University User on 05/17/2015
13. Christensen L. Normal and pathologic tissue reactions to soft tissue gel fillers.
REFERENCES Dermatol Surg. 2007;33(suppl 2):S168-S175.
14. Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L.
1. American Society of Plastic Surgeons. 2011 Plastic surgery procedural statistics. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011;
http://www.plasticsurgery.org/News-and-Resources/2011-Statistics-.html.Ac- 64(1):1-36.
cessed August 30, 2012. 15. Levy RM, Redbord KP, Hanke CW. Treatment of HIV lipoatrophy and lipoatro-
2. Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Der- phy of aging with poly-L-lactic acid: a prospective 3-year follow-up study. J Am
matol Surg. 2009;35(suppl 2):1672-1680. Acad Dermatol. 2008;59(6):923-933.
3. Emer J, Waldorf H. Injectable neurotoxins and fillers: there is no free lunch. Clin 16. Borelli C, Kunte C, Weisenseel P, Thoma-Greber E, Korting HC, Konz B. Deep
Dermatol. 2011;29(6):678-690. subcutaneous application of poly-L-lactic acid as a filler for facial lipoatrophy in
4. Cox SE. Clinical experience with filler complications. Dermatol Surg. 2009;35(suppl HIV-infected patients. Skin Pharmacol Physiol. 2005;18(6):273-278.
2):1661-1666. 17. Lemperle G, Morhenn VB, Charrier U. Human histology and persistence of vari-
5. Dayan SH, Arkins JP, Mathison CC. Management of impending necrosis asso-
ous injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg.
ciated with soft tissue filler injections. J Drugs Dermatol. 2011;10(9):1007-
2003;27(5):354-367.
1012.
18. Lam SM, Azizzadeh B, Graivier M. Injectable poly-L-lactic acid (Sculptra): tech-
6. Hirsch RJ, Cohen JL, Carruthers JD. Successful management of an unusual pre-
nical considerations in soft-tissue contouring. Plast Reconstr Surg. 2006;118
sentation of impending necrosis following a hyaluronic acid injection embolus
(3)(suppl):55S-63S.
and a proposed algorithm for management with hyaluronidase. Dermatol Surg.
19. Butterwick K, Lowe NJ. Injectable poly-L-lactic acid for cosmetic enhancement:
2007;33(3):357-360.
learning from the European experience. J Am Acad Dermatol. 2009;61(2):281-
7. Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: protocol
for prevention and treatment after use of dermal fillers. Dermatol Surg. 2006; 293.
32(2):276-281. 20. Sanofi-Aventis U.S. LLC. Sculptra (injectable poly-L-lactic acid) prescribing
8. Narins RS, Jewell M, Rubin M, Cohen J, Strobos J. Clinical conference: man- information. 2009. http://products.sanofi.us/sculptra/sculptra.html. Accessed Au-
agement of rare events following dermal fillers: focal necrosis and angry red bumps. gust 30, 2012.
Dermatol Surg. 2006;32(3):426-434. 21. Christensen L, Breiting V, Janssen M, Vuust J, Hogdall E. Adverse reactions to
9. Lambros V. The use of hyaluronidase to reverse the effects of hyaluronic acid injectable soft tissue permanent fillers. Aesthetic Plast Surg. 2005;29(1):34-
filler. Plast Reconstr Surg. 2004;114(1):277. 48.
10. Mertz PM. Cutaneous biofilms: friend or foe? Wounds. 2003;15(5):129-132. 22. Alcalay J, Alkalay R, Gat A, Yorav S. Late-onset granulomatous reaction to Artecoll.
11. Monheit GD, Rohrich RJ. The nature of long-term fillers and the risk of Dermatol Surg. 2003;29(8):859-862.
complications. Dermatol Surg. 2009;35(suppl 2):1598-1604. 23. Ghislanzoni M, Bianchi F, Barbareschi M, Alessi E. Cutaneous granulomatous
12. Jayaraman A, Wood TK. Bacterial quorum sensing: signals, circuits, and impli- reaction to injectable hyaluronic acid gel. Br J Dermatol. 2006;154(4):755-
cations for biofilms and disease. Annu Rev Biomed Eng. 2008;10:145-167. 758.
JAMA FACIAL PLAST SURG/ VOL 15 (NO. 3), MAY/JUNE 2013 WWW.JAMAFACIAL.COM
231
©2013 American Medical Association. All rights reserved.
Downloaded From: http://archfaci.jamanetwork.com/ by a New York University User on 05/17/2015
You might also like
- Complications After Cosmetic Periocular Filler Prevention and ManagementDocument13 pagesComplications After Cosmetic Periocular Filler Prevention and ManagementBruna BassoNo ratings yet
- Experience and Management of Intravascular Injection With Facial Fillers - Results of A Multinational Survey of Experienced InjectorsDocument7 pagesExperience and Management of Intravascular Injection With Facial Fillers - Results of A Multinational Survey of Experienced InjectorsKerlida SantosNo ratings yet
- Adverse Effects of FillersDocument9 pagesAdverse Effects of FillersElizabeth TovittoNo ratings yet
- ArtigoDocument12 pagesArtigoPaola PinheiroNo ratings yet
- Short-Term Treatment Outcomes of Facial RejuvenatiDocument8 pagesShort-Term Treatment Outcomes of Facial Rejuvenatihuyenthanh1807No ratings yet
- Complications-Of-Hyaluronic-Acid-Injections-Or-Som 2Document2 pagesComplications-Of-Hyaluronic-Acid-Injections-Or-Som 2umsztpcptdpolyroaeNo ratings yet
- JAMA Facial Plastic Surgery: Novel Use of A Volumizing Hyaluronic Acid Filler For Treatment of Infraorbital HollowsDocument23 pagesJAMA Facial Plastic Surgery: Novel Use of A Volumizing Hyaluronic Acid Filler For Treatment of Infraorbital HollowsDr. Hilder HernandezNo ratings yet
- Radiesse - Advanced Techniques and Applications For A Unique and Versatile ImplantDocument7 pagesRadiesse - Advanced Techniques and Applications For A Unique and Versatile ImplantAnonymous LnWIBo1G100% (2)
- Rhinoplasty - Indications and Techniques: Abel-Jan TasmanDocument23 pagesRhinoplasty - Indications and Techniques: Abel-Jan TasmanMohammad FareedNo ratings yet
- Sjy 013Document5 pagesSjy 013Ananda SantosNo ratings yet
- 2011 JAAD Vitiligo A Comprehensive OverviewDocument22 pages2011 JAAD Vitiligo A Comprehensive OverviewGOURINANDHANo ratings yet
- 1.9 Facial Dermal Fillers Selection of Appropriate Products and TechniquesDocument13 pages1.9 Facial Dermal Fillers Selection of Appropriate Products and TechniquesErik BrooksNo ratings yet
- 39-48 CMAC FinalDocument10 pages39-48 CMAC FinalAngela AguilarNo ratings yet
- Jacovella2006 - CópiaDocument13 pagesJacovella2006 - CópiaVanessa Müller BardiniNo ratings yet
- Management of Complications and Sequelae With Temporary Injectable FillersDocument8 pagesManagement of Complications and Sequelae With Temporary Injectable FillersPaola PinheiroNo ratings yet
- Superficial Dermal Fillers With Hyaluronic AcidDocument5 pagesSuperficial Dermal Fillers With Hyaluronic AcidchipanzeNo ratings yet
- B Evolution - of - Facial - Aesthetic - Treatment - Over - Five.4Document9 pagesB Evolution - of - Facial - Aesthetic - Treatment - Over - Five.4vmx45ncwd7No ratings yet
- Skin Beauty InjectionsDocument12 pagesSkin Beauty Injectionst3kla100% (1)
- Cosmetic Special Topic: Practice Advisory On LiposuctionDocument13 pagesCosmetic Special Topic: Practice Advisory On LiposuctionLuis AlcantaraNo ratings yet
- Midface Clinical Anatomy and Regional Approaches.31Document17 pagesMidface Clinical Anatomy and Regional Approaches.31Andreas Chandra100% (3)
- Alam2018 Fillers and NeurotoxinsDocument13 pagesAlam2018 Fillers and NeurotoxinsbaranNo ratings yet
- Adverse Effects of Fillers and TheirDocument16 pagesAdverse Effects of Fillers and Theirt3klaNo ratings yet
- Risk Factors in Bone Augmentation Procedures: Peter K. Moy - Tara AghalooDocument15 pagesRisk Factors in Bone Augmentation Procedures: Peter K. Moy - Tara Aghalooxiaoxin zhangNo ratings yet
- A Review of Dermal Fillers in Facial Plastic SurgeDocument9 pagesA Review of Dermal Fillers in Facial Plastic Surgesadia Gul - Oral and Maxilofacial SurgeryNo ratings yet
- Global Aesthetics Consensus Avoidance and ManagemeDocument12 pagesGlobal Aesthetics Consensus Avoidance and ManagemednaielNo ratings yet
- Choi2015 Sculptra Sulco NasoDocument10 pagesChoi2015 Sculptra Sulco Nasoana clara scopelNo ratings yet
- Wide-Awake Local Anesthesia With No Tourniquet AnDocument11 pagesWide-Awake Local Anesthesia With No Tourniquet Andaniel saucedoNo ratings yet
- Décolletage: Regional Approaches With Injectable FillersDocument6 pagesDécolletage: Regional Approaches With Injectable Fillersdanielagonzalezt5No ratings yet
- LiposuctionDocument8 pagesLiposuctionnikitagustiNo ratings yet
- Cap 158 COSMETIC SURGERYDocument24 pagesCap 158 COSMETIC SURGERYalexxa_1900No ratings yet
- Art I Go Lift TechniqueDocument4 pagesArt I Go Lift Techniquejoann15marrieNo ratings yet
- Course Materials On WoundsDocument11 pagesCourse Materials On WoundsP.Senthil SelvamNo ratings yet
- MichDocument7 pagesMichcinthiakarenhernandez461No ratings yet
- OZTURK - Complications Following Injection of SOFT-TISSUE FILLERDocument16 pagesOZTURK - Complications Following Injection of SOFT-TISSUE FILLERdeliveryworkNo ratings yet
- 28 1561634191Document5 pages28 1561634191Trần Duy TânNo ratings yet
- Pi Is 0190962217328724Document3 pagesPi Is 0190962217328724Muhammad Irfan AnwarNo ratings yet
- Complications of Nonpermanent Facial Fillers - A Systematic ReviewDocument17 pagesComplications of Nonpermanent Facial Fillers - A Systematic ReviewKerlida SantosNo ratings yet
- JERD FAHL ClassVDocument19 pagesJERD FAHL ClassVElly FatmaNo ratings yet
- Quality of Life Assessment of Patients Utilizing Orbital Implant Supported ProsthesesDocument6 pagesQuality of Life Assessment of Patients Utilizing Orbital Implant Supported ProsthesesBagis Emre GulNo ratings yet
- J of Cosmetic Dermatology - 2021 - Chuang - Cephalometric Analysis Following Combined Sub SMAS Hyaluronic Acid InjectionDocument8 pagesJ of Cosmetic Dermatology - 2021 - Chuang - Cephalometric Analysis Following Combined Sub SMAS Hyaluronic Acid Injectionjoann15marrieNo ratings yet
- Soft Tissue Augmentation With Dermal Fillers, Part 1: Lips and Lower FaceDocument6 pagesSoft Tissue Augmentation With Dermal Fillers, Part 1: Lips and Lower FaceMichele CarvalhoNo ratings yet
- Comparing Cannula Based Subcision With The Common Needle Method: A Clinical TrialDocument7 pagesComparing Cannula Based Subcision With The Common Needle Method: A Clinical Trial郭先薈No ratings yet
- Complications With Graft HarvestingDocument12 pagesComplications With Graft HarvestingDr. DeeptiNo ratings yet
- The International Journal of Periodontics & Restorative DentistryDocument6 pagesThe International Journal of Periodontics & Restorative DentistryDr. DeeptiNo ratings yet
- Vascular Complications After Facial Filler Injection:: A Literature Review and Meta-AnalysisDocument9 pagesVascular Complications After Facial Filler Injection:: A Literature Review and Meta-AnalysisIsabel JiménezNo ratings yet
- Bruggmann2010 IntraabdomadhesionsDocument10 pagesBruggmann2010 IntraabdomadhesionsQuiroprácticaParaTodosNo ratings yet
- Pmfaas20 ComplicationsDocument3 pagesPmfaas20 ComplicationsandreinacssilvaNo ratings yet
- Volumizacao Com PreenchedoresDocument18 pagesVolumizacao Com PreenchedoresMichele CarvalhoNo ratings yet
- Undisturbedhealing JWCRipponetalDocument9 pagesUndisturbedhealing JWCRipponetalRachel RiordanNo ratings yet
- View Doc 635470672036959908Document6 pagesView Doc 635470672036959908abhishekbmcNo ratings yet
- Hyaluronidase ConsensusDocument6 pagesHyaluronidase ConsensusyasinoNo ratings yet
- Effectiveness Using of Transparent Film Dressing As Skin Barrier Protection To Prevent Maceration in The Wound Care Process at Bilqiss Medika Clinic Bekasi - West Java, IndonesiaDocument12 pagesEffectiveness Using of Transparent Film Dressing As Skin Barrier Protection To Prevent Maceration in The Wound Care Process at Bilqiss Medika Clinic Bekasi - West Java, IndonesiaInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Interventions To Decrease Catheter-Related Bloodstream Infections in The ICU: The Keystone Intensive Care Unit ProjectDocument5 pagesInterventions To Decrease Catheter-Related Bloodstream Infections in The ICU: The Keystone Intensive Care Unit ProjectanggitaNo ratings yet
- Iverson 2008Document11 pagesIverson 2008Bruno MañonNo ratings yet
- Face Transp 1Document6 pagesFace Transp 1Jorge Muñoz Cirujano PlásticoNo ratings yet
- Nonsurgical Periodontal Therapy in 2000:A Literature Review: Article 2Document13 pagesNonsurgical Periodontal Therapy in 2000:A Literature Review: Article 2SofiaNo ratings yet
- Preventing Breast Implant ContaminationDocument4 pagesPreventing Breast Implant Contaminationvictorubong404No ratings yet
- Salvaging The Unavoidable: A Review of Complications in Pediatric Tissue ExpansionDocument10 pagesSalvaging The Unavoidable: A Review of Complications in Pediatric Tissue Expansionsmansa123No ratings yet
- Urdialesglvez 2017Document11 pagesUrdialesglvez 2017Armin ArceoNo ratings yet
- Successfully Managing Impending Skin Necrosis Following Hyaluronic Acid Filler Injection, Using High-Dose Pulsed HyaluronidaseDocument3 pagesSuccessfully Managing Impending Skin Necrosis Following Hyaluronic Acid Filler Injection, Using High-Dose Pulsed HyaluronidaseArmin ArceoNo ratings yet
- Beware What You Inject: Complications of Injectables-Dermal FillersDocument7 pagesBeware What You Inject: Complications of Injectables-Dermal FillersArmin ArceoNo ratings yet
- Late Onset Inflammatory Response To Hyaluronic.4Document7 pagesLate Onset Inflammatory Response To Hyaluronic.4Armin ArceoNo ratings yet
- Injectable Facial Fillers: Imaging Features, Complications, and Diagnostic Pitfalls at MRI and PET CTDocument16 pagesInjectable Facial Fillers: Imaging Features, Complications, and Diagnostic Pitfalls at MRI and PET CTArmin ArceoNo ratings yet
- Consensus Statement On Prevention and Management of Adverse Effects Following Rejuvenation Procedures With Hyaluronic Acid Based FillersDocument26 pagesConsensus Statement On Prevention and Management of Adverse Effects Following Rejuvenation Procedures With Hyaluronic Acid Based FillersArmin ArceoNo ratings yet
- Efficacy of Retrobulbar Hyaluronidase Injection For Vision Loss Resulting From Hyaluronic Acid Filler EmbolizationDocument11 pagesEfficacy of Retrobulbar Hyaluronidase Injection For Vision Loss Resulting From Hyaluronic Acid Filler EmbolizationArmin ArceoNo ratings yet
- Complication Management Following Rejuvenation.98146Document11 pagesComplication Management Following Rejuvenation.98146Armin ArceoNo ratings yet
- An Overview of Permanent and Semipermanent Fillers: SummaryDocument8 pagesAn Overview of Permanent and Semipermanent Fillers: SummaryArmin ArceoNo ratings yet
- Jong Woo Choi, MD, PHD Woo Shik Jeong, MD Sin Young Sang, MD Kyung S. Koh, MD, PHDDocument2 pagesJong Woo Choi, MD, PHD Woo Shik Jeong, MD Sin Young Sang, MD Kyung S. Koh, MD, PHDArmin ArceoNo ratings yet
- Sarcopenic Obesity, Insulin Resitance and Their Implications in Cardiovascular and Metabolic ConsequencesDocument17 pagesSarcopenic Obesity, Insulin Resitance and Their Implications in Cardiovascular and Metabolic ConsequencesArmin ArceoNo ratings yet
- Mesotherapy in The Treatment of MusculoskeletalDocument11 pagesMesotherapy in The Treatment of MusculoskeletalArmin ArceoNo ratings yet
- Zhuangzi's Butterfly DreamDocument4 pagesZhuangzi's Butterfly DreamUnnamed EntityNo ratings yet
- Bai Tap On Tap Unit 123Document8 pagesBai Tap On Tap Unit 123Le VanNo ratings yet
- Loading Ericsson GPEH Files - Into ANALYZER - Tech NotesDocument9 pagesLoading Ericsson GPEH Files - Into ANALYZER - Tech Notesbmaia18No ratings yet
- Hci - Web Interface DesignDocument54 pagesHci - Web Interface DesigngopivrajanNo ratings yet
- Construction Method Statement SafetyDocument4 pagesConstruction Method Statement SafetyRaiZaNo ratings yet
- Tinynet (Mytyvm) : Creating Virtual MachinesDocument38 pagesTinynet (Mytyvm) : Creating Virtual MachinesSayyam ChNo ratings yet
- BañadosHenneaux-1993-Geometry of The 2+1 Black HoleDocument52 pagesBañadosHenneaux-1993-Geometry of The 2+1 Black HoleGuido FranchettiNo ratings yet
- Data Science Tech Cover Letter TemplateDocument1 pageData Science Tech Cover Letter Templatemaqbool bhaiNo ratings yet
- Companion Study Guide: 15 LessonsDocument124 pagesCompanion Study Guide: 15 LessonsLuisk MaldonadoNo ratings yet
- CASE OF VRZIC v. CROATIADocument22 pagesCASE OF VRZIC v. CROATIAVeronica PozneacovaNo ratings yet
- Is Your: WritingDocument84 pagesIs Your: WritingVito a sapada Arung wetteNo ratings yet
- Las - 9-12Document11 pagesLas - 9-12maria teresa aparreNo ratings yet
- Lab 2 (Methods, Classes)Document23 pagesLab 2 (Methods, Classes)Skizzo PereñaNo ratings yet
- Fast Feng ShuiDocument250 pagesFast Feng Shuimd91101No ratings yet
- Barrios V MartinezDocument13 pagesBarrios V MartinezMp CasNo ratings yet
- Weidner - Fulcanelli's Final RevelationDocument22 pagesWeidner - Fulcanelli's Final Revelationjosnup100% (3)
- Finance Notes at Mba FinanceDocument40 pagesFinance Notes at Mba FinanceBabasab Patil (Karrisatte)No ratings yet
- Respiratory SystemDocument9 pagesRespiratory Systemtheglobalnursing89% (9)
- Jun 2003 - Qns Mod BDocument13 pagesJun 2003 - Qns Mod BHubbak KhanNo ratings yet
- Podar School HomeworkDocument7 pagesPodar School Homeworkerr64wxh100% (1)
- An Investigation Into The Use of GAMA Water Tunnel For Visualization of Vortex Breakdown On The Delta WingDocument7 pagesAn Investigation Into The Use of GAMA Water Tunnel For Visualization of Vortex Breakdown On The Delta WingEdy BudimanNo ratings yet
- Whare Group Final PresentationDocument19 pagesWhare Group Final PresentationAruna MadasamyNo ratings yet
- List HargaDocument28 pagesList HargaFitra noviandaNo ratings yet
- 2012 Movie SummaryDocument2 pages2012 Movie SummaryRenée NinteNo ratings yet
- 3306 Mayfair ST.: RFI'sDocument9 pages3306 Mayfair ST.: RFI'sRobbieMaxwellNo ratings yet
- AP Macroeconomics Review Sheet 2013Document7 pagesAP Macroeconomics Review Sheet 2013Crystal Farmer100% (4)
- Experimentation, Orgasms, and The Rise of Anal Sex. - by William Saletan - SDocument13 pagesExperimentation, Orgasms, and The Rise of Anal Sex. - by William Saletan - Saweawerwerwe100% (1)
- Santayana vs. Alampay, A.C. No. 5878, March 21, 2005Document2 pagesSantayana vs. Alampay, A.C. No. 5878, March 21, 2005Charles Roger Raya50% (2)
- h18 Philippines1Document16 pagesh18 Philippines1Stephanie Mae RadamNo ratings yet
- STT Tecnica QuirúrgicaDocument5 pagesSTT Tecnica QuirúrgicaNathaly GuevaraNo ratings yet