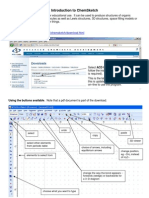
Professional Documents
Culture Documents
Aspirin Level 4: Gulshad Ahmad Aims
Aspirin Level 4: Gulshad Ahmad Aims
Uploaded by
mjunaidOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aspirin Level 4: Gulshad Ahmad Aims
Aspirin Level 4: Gulshad Ahmad Aims
Uploaded by
mjunaidCopyright:
Available Formats
Aspirin
level 4
GULSHAD AHMAD
Aims
In level 4, you'll find out about synthesising aspirin using different reagents. You will use thin layer chromatography
(TLC) to identify which reactants produce aspirin and decide the best reaction to produce aspirin on an industrial scale.
In each activity you'll be able to collect points. At the end of the level you can restart to improve your skills.
Video 100 Points
Comprehension 70 Points 4 Attempts
Ethanoic anhydride is the electrophilic reagent in aspirin synthesis.
Ethanoic anhydride can be replaced with a different reagent when making aspirin.
When an alternative electrophilic reagent is used to make aspirin the leaving group changes.
When ethanoic anhydride is replaced with a more reactive reagent it reduces the reaction time.
To make industrial production as environmentally friendly as possible energy use should be minimised.
Reactants 50 Points 5 Attempts
Ethanoic anhydride and ethanoyl chloride can both be used to make aspirin because they both have good enough
leaving groups to undergo esterification. The formation of aspirin can be seen by TLC (A: 2-Hydroxybenzoic acid, B:
Aspirin).
Leaving groups 50 Points 5 Attempts
For ethanoic anhydride the leaving group is an ethanoate ion and for ethanoyl chloride the leaving group is a chloride
ion. Good leaving groups have electronegative atoms and must form stable anions.
Reagents 50 Points 5 Attempts
Ethanoic anhydride is chosen over ethanoyl chloride for industrial production of aspirin because:
ethanoic anhydride is less hazardous than ethanoyl chloride
ethanoic anhydride is cheaper.
Review
Things I did well on:
Things I could improve on:
You have identified ethanoic anhydride as the best reagent for the industrial scale synthesis of aspirin. Can you
remember why?
You might also like
- Determination of Fluoride Concentration Using Ion Selective ElectrodeDocument7 pagesDetermination of Fluoride Concentration Using Ion Selective ElectrodeAmanda WangNo ratings yet
- PAG 10.3 Learner SheetDocument3 pagesPAG 10.3 Learner SheetJoshna JoseNo ratings yet
- Aldol CondensationDocument5 pagesAldol CondensationKatherine McLarneyNo ratings yet
- Vapor-Liquid Equilibria For The Ternary System Methyl Acetate-Benzene-CyclohexaneDocument6 pagesVapor-Liquid Equilibria For The Ternary System Methyl Acetate-Benzene-CyclohexaneDaniel DominguezNo ratings yet
- Diffusion Effects in Enzymes Immobilized in A Porous MatrixDocument18 pagesDiffusion Effects in Enzymes Immobilized in A Porous MatrixAegee0% (1)
- Exp 7 (Solved)Document9 pagesExp 7 (Solved)mahmudul100% (1)
- Answer For TutorialDocument7 pagesAnswer For TutorialFatur RohimNo ratings yet
- Copper Determination in Water by Standard Addition PotentiometryDocument4 pagesCopper Determination in Water by Standard Addition PotentiometryAura Ballesteros MontealegreNo ratings yet
- Solid-Liquid Extraction Leaching: ChapterDocument15 pagesSolid-Liquid Extraction Leaching: Chapterاحمد حمید کارسول عزیزNo ratings yet
- AOAC 931-08 Formaldeyde in FoodDocument1 pageAOAC 931-08 Formaldeyde in FoodJocilene DantasNo ratings yet
- Partial Molar VolumeDocument6 pagesPartial Molar VolumeAaron Chris GonzalesNo ratings yet
- RMN ProblemsDocument7 pagesRMN ProblemsAnonymous llSDP0tNo ratings yet
- CPP Assignment 1Document2 pagesCPP Assignment 1AmandaEdwinNo ratings yet
- Exp 8 (Solved)Document11 pagesExp 8 (Solved)mahmudulNo ratings yet
- C01 14PDocument33 pagesC01 14PTiffany LiuNo ratings yet
- The Sadtler Handbook of Proton NMR Spectra PDFDocument297 pagesThe Sadtler Handbook of Proton NMR Spectra PDFFedegos_toso100% (1)
- CHEMSKETCHDocument10 pagesCHEMSKETCHGeliane RochaNo ratings yet
- Tutorial 4 Achem PDFDocument12 pagesTutorial 4 Achem PDFyassinroslanNo ratings yet
- Centripetal Acceleration Lab ReportDocument7 pagesCentripetal Acceleration Lab Reportapi-263389150No ratings yet
- Orgo Cheat Sheets 08 2019 PDFDocument34 pagesOrgo Cheat Sheets 08 2019 PDFKobe AcobNo ratings yet
- Co (NH3) 6Document1 pageCo (NH3) 6Ayotunde OnasanyaNo ratings yet
- Auto Catalytic Reactions PresentationDocument15 pagesAuto Catalytic Reactions PresentationAmad AmadNo ratings yet
- FLASH ADIABÁTICO - Separation Process Principles - SEADER - 3rdedDocument5 pagesFLASH ADIABÁTICO - Separation Process Principles - SEADER - 3rdedMaykkkowNo ratings yet
- Exp 6 (Solved) 1Document8 pagesExp 6 (Solved) 1mahmudulNo ratings yet
- D2087 - 06 (2012) Standard Test Method For Iron in Formaldehyde Solutions PDFDocument3 pagesD2087 - 06 (2012) Standard Test Method For Iron in Formaldehyde Solutions PDFJacques BlueqNo ratings yet
- PDC Compilation PDFDocument87 pagesPDC Compilation PDFScrappy WellNo ratings yet
- HW Set 1Document6 pagesHW Set 1GsusKrystNo ratings yet
- Hunter NashDocument14 pagesHunter NashSata AjjamNo ratings yet
- Energy Balances On Reactive ProcessDocument12 pagesEnergy Balances On Reactive ProcessKhairun Niesa100% (1)
- LAB GravimetricAnalysisSulfateMixtureDocument3 pagesLAB GravimetricAnalysisSulfateMixtureDNo ratings yet
- A. Experiment Title: The Making of N-Butyl Acetate B. Experiment Started Date: Wednesday, March 4Document21 pagesA. Experiment Title: The Making of N-Butyl Acetate B. Experiment Started Date: Wednesday, March 4Era MelaniaNo ratings yet
- Unit - IV: Stereochemistry, Reaction Mechanism and Synthesis of Drug MoleculesDocument8 pagesUnit - IV: Stereochemistry, Reaction Mechanism and Synthesis of Drug MoleculesSai shravya GorekarNo ratings yet
- Solomon's Chapter 15 SolutionDocument34 pagesSolomon's Chapter 15 SolutionRobert0% (2)
- Quiz 4Document44 pagesQuiz 4Juba W Allen50% (4)
- Electrochemistry Class 12 NotesDocument53 pagesElectrochemistry Class 12 NotesGirish Arora0% (1)
- Autonomous Maze Solving Robot Using Arduino: A Project Seminar Report OnDocument18 pagesAutonomous Maze Solving Robot Using Arduino: A Project Seminar Report OnAkhilaNo ratings yet
- Adama Science and Technology University School of Applied Natural Science Department of Applied MathematicsDocument9 pagesAdama Science and Technology University School of Applied Natural Science Department of Applied MathematicsALEMAYEHUNo ratings yet
- Sheet - 01 (Solution) - Liquid ExerciseDocument44 pagesSheet - 01 (Solution) - Liquid Exercisejalpatel71001100% (1)
- Acetaldol MsdsDocument6 pagesAcetaldol Msdsdlr1233No ratings yet
- Bromination Acetanilide W2014Document4 pagesBromination Acetanilide W2014zzdantezzNo ratings yet
- 2.02 Determination of The Formula of A Complex by SpectrophotometryDocument5 pages2.02 Determination of The Formula of A Complex by Spectrophotometrycahyoaam100% (1)
- Lab 6: Base Extraction of Benzoic Acid From Acetanilide Followed by Recrystallization and MP DeterminationDocument9 pagesLab 6: Base Extraction of Benzoic Acid From Acetanilide Followed by Recrystallization and MP Determinationbebo4gpaNo ratings yet
- 3 Steady State DiffusionDocument25 pages3 Steady State DiffusionShahadat AwanNo ratings yet
- Chapter 11. The Group 2 ElementsDocument13 pagesChapter 11. The Group 2 ElementsramNo ratings yet
- Test 3 Solution 2010 PDFDocument4 pagesTest 3 Solution 2010 PDFManishaa Varatha RajuNo ratings yet
- Online Activity - CalorimetryDocument3 pagesOnline Activity - Calorimetryapi-617652463No ratings yet
- The Fluoride Ion Selective Electrode ExperimentDocument5 pagesThe Fluoride Ion Selective Electrode Experimentlisaaliyo0% (1)
- The Calorimetric Method of Determining The IntegralDocument3 pagesThe Calorimetric Method of Determining The IntegralLoveFreequencyNo ratings yet
- CH 06Document71 pagesCH 06نزار الدهاميNo ratings yet
- Set6ans 10Document4 pagesSet6ans 10Natália FerreiraNo ratings yet
- Reaction Second Order - Alkaline Hydrolysis of EsterDocument3 pagesReaction Second Order - Alkaline Hydrolysis of EsterFe Sh100% (1)
- Operator Lesson PowerPoint PDFDocument41 pagesOperator Lesson PowerPoint PDFJoseNo ratings yet
- Chem 373 - Lecture 32: The Hückel MethodDocument26 pagesChem 373 - Lecture 32: The Hückel MethodNuansak3100% (1)
- LAB5Document1 pageLAB5Tarmizi Al-AminNo ratings yet
- Synthesis of Na (Fe (EDTA) ) ×3H2ODocument2 pagesSynthesis of Na (Fe (EDTA) ) ×3H2OslayercroNo ratings yet
- Organic Comp - Distinguish TestsDocument1 pageOrganic Comp - Distinguish TestsKatniss TathagataNo ratings yet
- Absorption Laws (Quantitative Analysis)Document15 pagesAbsorption Laws (Quantitative Analysis)Belay HaileNo ratings yet
- Aspirin Level 2: Gulshad Ahmad AimsDocument3 pagesAspirin Level 2: Gulshad Ahmad AimsmjunaidNo ratings yet
- The Synthesis of A Medicinal Agent-AspirinDocument5 pagesThe Synthesis of A Medicinal Agent-AspirinD ROYNo ratings yet
- Synthesis of Acetylsalicylic Acid (Aspirin)Document7 pagesSynthesis of Acetylsalicylic Acid (Aspirin)Nor Ashikin IsmailNo ratings yet
- Formula'sDocument1 pageFormula'smjunaidNo ratings yet
- Ayyaz CVDocument1 pageAyyaz CVmjunaidNo ratings yet
- Oil MovementDocument3 pagesOil MovementmjunaidNo ratings yet
- National Refinery Limited ShkjyukjyukyDocument29 pagesNational Refinery Limited ShkjyukjyukymjunaidNo ratings yet
- Q Chem CVDocument2 pagesQ Chem CVmjunaidNo ratings yet
- National Refinery Limited 2Document21 pagesNational Refinery Limited 2mjunaidNo ratings yet
- Yaseen Khan: 2017-MC-312Document4 pagesYaseen Khan: 2017-MC-312mjunaidNo ratings yet
- Akhtar SaeedDocument3 pagesAkhtar SaeedmjunaidNo ratings yet
- Reid Vapor Pressure MeterDocument3 pagesReid Vapor Pressure MetermjunaidNo ratings yet
- Exp 3Document15 pagesExp 3mjunaidNo ratings yet
- 1 2016-MC-312Document4 pages1 2016-MC-312mjunaidNo ratings yet
- Department of Chemical and Polymer Engineering - Risk AssessmentDocument4 pagesDepartment of Chemical and Polymer Engineering - Risk AssessmentmjunaidNo ratings yet
- Assignment # 2: Subject Name Reg# ClassDocument10 pagesAssignment # 2: Subject Name Reg# ClassmjunaidNo ratings yet
- Energy Engineering Lab. AssignmentDocument1 pageEnergy Engineering Lab. AssignmentmjunaidNo ratings yet
- Wetted Wall ColumnDocument7 pagesWetted Wall ColumnmjunaidNo ratings yet
- Chemical Engineering Plant DesignDocument15 pagesChemical Engineering Plant DesignmjunaidNo ratings yet
- Experiment No 1 (Tray Dryer)Document8 pagesExperiment No 1 (Tray Dryer)mjunaidNo ratings yet
- Department of Chemical and Polymer Engineering - Risk AssessmentDocument4 pagesDepartment of Chemical and Polymer Engineering - Risk AssessmentmjunaidNo ratings yet
- Department of Chemical and Polymer Engineering - Risk AssessmentDocument4 pagesDepartment of Chemical and Polymer Engineering - Risk AssessmentmjunaidNo ratings yet
- Experiment No #2: ObjectiveDocument5 pagesExperiment No #2: ObjectivemjunaidNo ratings yet
- Ammonium Nitrate The Stengel Process: J. DorseyDocument7 pagesAmmonium Nitrate The Stengel Process: J. DorseymjunaidNo ratings yet
- Lab Report: University of Enginering and Techonology, LahoreDocument20 pagesLab Report: University of Enginering and Techonology, LahoremjunaidNo ratings yet
- Stengel Vs PrilledDocument1 pageStengel Vs PrilledmjunaidNo ratings yet
- Assignment # 1: Subject Class Reg# Submitted To: Submitted byDocument7 pagesAssignment # 1: Subject Class Reg# Submitted To: Submitted bymjunaidNo ratings yet
- Lab Report: Instrumentation and ControlDocument7 pagesLab Report: Instrumentation and ControlmjunaidNo ratings yet
- Lecture1 HumidifictionDocument20 pagesLecture1 HumidifictionmjunaidNo ratings yet
- Ammonium Nitrate Vs Urea: ReferenceDocument2 pagesAmmonium Nitrate Vs Urea: ReferencemjunaidNo ratings yet