Professional Documents
Culture Documents
Lab 4 - Titration Curves - Lab Manual
Uploaded by
Roberto BeltOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lab 4 - Titration Curves - Lab Manual
Uploaded by
Roberto BeltCopyright:
Available Formats
Chemistry 163 Lab
TITRATION CURVES
Acid-base titration is a useful technique for determining the concentration of an acidic or
basic solution. As you will see today, titration of a weak acid or base can also be used to
determine the pKa or pKb for the acid/base. You have already been introduced to titration
technique in previous labs. Today you will use a pH meter connected to a computer to
generate a titration curve. A titration curve shows how the pH of a solution changes as
acid or base is added. The shape of a titration curve is influenced by both the
concentration and structure of the acid. You will see two different titration curves in
today's lab.
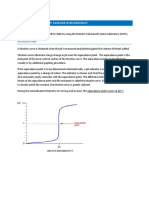
A typical strong acid-strong base titration curve is shown in Figure 1 below. In this lab
you will be titrating weak acids which look a bit different, but still have the steep section
in the middle. In previous titration experiments you have determined the equivalence
point of the titration by observing the color change of an indicator. When using a pH
meter to monitor the titration, the endpoint is determined by the inflection point of the
curve. This provides a more accurate determination of the equivalence point. The
inflection point is the steepest part of the curve, and is also the point on the curve where
the curvature changes. In calculus terms, this is the point where the second derivative
equals zero.
Figure 1: Strong acid-strong base titration curve
The LoggerPro program that you will be using to collect the data has a feature which will
allow you to calculate and plot the second derivative of pH as a function of volume of
base added. There is a video you can watch which goes over how to make this plot and
find the endpoint.
In this lab you will work with a partner to complete two titrations. First you will titrate a
weak acid (KHP) with a ~0.1 M sodium hydroxide solution. From the results of this
Edmonds Community College
Page 1 of 4
Chemistry 163 Lab
titration you will be able to calculate the exact concentration of the NaOH. (In other
words, you will standardize the NaOH. Do you remember why this is necessary?) Next
you will titrate an unknown polyprotic acid. From your titration data, you will be able to
calculate the acid ionization constants and the molecular weight of the unknown acid.
A pH meter will be used to measure the pH. So that you can monitor each titration in real
time, you will enter and plot the data on a computer as the titration proceeds.
Part I. Titration of a known monoprotic weak acid (KHP)
The objectives of this titration are to:
(1) observe the titration curve for a weak acid with a strong base
(2) use the data collected to determine the exact molarity of the NaOH
(3) find the pKa for KHP
Part II. Titration of an unknown polyprotic acid
The objectives of this titration are to:
(1) observe the titration curve for a weak polyprotic acid with a strong base
(2) to use the data collected to determine the molar mass of the acid
(3) to find the pKa values for the polyprotic acid.
Before coming to lab…..
● Read the lab thoroughly and prepare your notebook.
● Review your text for a further discussion of titration curves, particularly the
discussion of titrations involving weak acids and bases.
● Look up and record the molar mass and structure of KHP. Record these in your
notebook.
● Review how to calculate the molarity of an acid or base from the endpoint of a
titration. You may wish to review the Standardization of NaOH lab from
Chemistry 161.
● Preview the posted video which shows how to use the LoggerPro program to
make a second derivative plot.
Edmonds Community College
Page 2 of 4
Chemistry 163 Lab
Directions
1. Set up a titration apparatus as shown below. Position the pH electrode in the
solution and adjust its position so that it is not struck by the stirring bar.
2. Connect a pH electrode to channel 1 of a LabQuest interface and connect the
interface to a computer. Open the LoggerPro program. Open the folder Chemistry
with Vernier and from there select the Acid-Base Titrations Experiment. The axes
should be properly scaled for your experiment.
3. Use the buffer solutions to calibrate the pH probe.
4. Set up a buret with the NaOH solution provided. Fill to the 0.00 mL line.
5. You will be given a vial containing KHP. Dissolve the entire sample in about 40
mL of water and determine the exact mass by weighing the vial before and after.
6. Add a few drops of phenolphthalein to the acid. This is not necessary because you
will be finding the equivalence point from the graph, but it is useful to observe
how the equivalence point you get from the graph compares to what you would
determine using the indicator.
7. In your notebook, calculate the expected endpoint based on the mass of KHP
used.
Edmonds Community College
Page 3 of 4
Chemistry 163 Lab
8. Record the initial pH of the acid solution. This is the pH with no NaOH added.
You will be entering data manually as you titrate. Record the initial volume (0.00
mL) of NaOH in the buret. As you continue to enter data, the titration curve will
be displayed on the screen.
9. Begin titrating with NaOH. You should try to produce a pH change of
approximately 0.2 units with each addition of base. If the pH changes too
rapidly, your curves will not be smooth and you will have difficulty determining
the equivalence point. Do not worry if you “overshoot” a change of 0.2 pH units,
but be sure to record the exact volumes and pH values.
10. For each addition of base, record the exact volume of NaOH added and the exact
pH. Remember that burets should be read to the hundredths place. Note: When
the pH begins to change rapidly you will need to add base in much smaller
increments in order to produce a change of only 0.2 units.
11. Record the volume of base and the pH at the point when the solution turns pink.
You do not need this information for your calculations, but it will be interesting to
observe how or if the two methods for determining the equivalence point differ.
12. Continue titrating to beyond the equivalence point, where the curve begins to
flatten out.
13. Add a column to your data table to calculate the second derivative of the data and
plot this data to determine the equivalence point. Review the posted video for how
to do this. Save the file to your ACS account or to a removable drive. Do not save
to the hard drive or your data will be lost.
14. Repeat the steps above with your sample of an unknown polyprotic acid. Record
the unknown number. Remember that you will have two equivalence points. If
you add base too quickly, you may miss the first one.
Calculations and Post Lab
Your post lab template is available on Canvas. For your post lab you will:
1. Calculate the exact molarity of the NaOH
2. Find the pKa for KHP
3. Find the molar mass of your unknown polyprotic acid
4. Find the pKa values for the polyprotic acid
Before completing your post lab you must submit your results to the Google Form for
checking.
Edmonds Community College
Page 4 of 4
You might also like
- A Laboratory Manual of Physical PharmaceuticsFrom EverandA Laboratory Manual of Physical PharmaceuticsRating: 2.5 out of 5 stars2.5/5 (2)
- Preparative Chromatography for Separation of ProteinsFrom EverandPreparative Chromatography for Separation of ProteinsArne StabyNo ratings yet
- Acid/Base Titration LabDocument5 pagesAcid/Base Titration LabDavid GrahamNo ratings yet
- pH Titration Soft DrinksDocument7 pagespH Titration Soft DrinksLakshmankumar TjpsNo ratings yet
- Experiment 4 PDFDocument7 pagesExperiment 4 PDFsaiNo ratings yet
- Determine Phosphoric Acid Content in ColaDocument10 pagesDetermine Phosphoric Acid Content in ColaMaMtNo ratings yet
- PH Titration LabQuest PDFDocument3 pagesPH Titration LabQuest PDFAntónio MatiasNo ratings yet
- Determination of pKa for Weak AcidDocument5 pagesDetermination of pKa for Weak AcidSonu DubeyNo ratings yet
- Potentiometric Titration of a Weak AcidDocument14 pagesPotentiometric Titration of a Weak AcidMay LeeNo ratings yet
- Determine Ka of Acetic Acid Using Half-TitrationDocument4 pagesDetermine Ka of Acetic Acid Using Half-TitrationSung Hoon ParkNo ratings yet
- Acid Base Laboratory StudentsDocument14 pagesAcid Base Laboratory StudentsAdriana Flores DepazNo ratings yet
- Acid Base Titration Experiment 4Document10 pagesAcid Base Titration Experiment 4pokesurfer100% (3)
- 06 and 07 Standardization of NaOH and Acid Base TitrationDocument16 pages06 and 07 Standardization of NaOH and Acid Base TitrationTyler Hardy80% (5)
- Titration Notes: MethodDocument3 pagesTitration Notes: MethodArSlanRahatNo ratings yet
- Analyze Soda AshDocument6 pagesAnalyze Soda AshyzzacamilleaNo ratings yet
- Mixture of Carbonate BicarbonateDocument9 pagesMixture of Carbonate BicarbonateIan Justine SanchezNo ratings yet
- ABcurves SP 19Document8 pagesABcurves SP 19Sakshi BangarwaNo ratings yet
- AP Chemistry Investigation 4 - Judy, Paul, AnthonyDocument13 pagesAP Chemistry Investigation 4 - Judy, Paul, AnthonyAnthony HowerNo ratings yet
- CHEM 161 Titration Practice For Sp2021Document7 pagesCHEM 161 Titration Practice For Sp2021いちごだいふくNo ratings yet
- pH Titration Lab Experiment Ka DeterminationDocument4 pagespH Titration Lab Experiment Ka DeterminationxmusiqaNo ratings yet
- S Determination of Phosphoric Acid Content in SoftdrinksDocument5 pagesS Determination of Phosphoric Acid Content in SoftdrinksMike Anderson0% (1)
- Potentiometric TitrationDocument9 pagesPotentiometric Titrationiah_guevarraNo ratings yet
- Analytical Chemistry Ii SCH2106 PDFDocument13 pagesAnalytical Chemistry Ii SCH2106 PDFAndrew May NcubeNo ratings yet
- Weak Acid Strong Base Titration LabDocument8 pagesWeak Acid Strong Base Titration Labapi-265089380100% (1)
- Determining The Amount of Acetic Acid in VinegarDocument2 pagesDetermining The Amount of Acetic Acid in VinegarAadhi JNo ratings yet
- ProjectDocument2 pagesProjectCindy Mae A. PogoyNo ratings yet
- 12 - Clear Soda Titration Lab 2023Document4 pages12 - Clear Soda Titration Lab 2023Mathar BashirNo ratings yet
- NSCI/NENG 115 Chemical Principles LabDocument7 pagesNSCI/NENG 115 Chemical Principles LabIsaac SnitkoffNo ratings yet
- PH and BuffersDocument4 pagesPH and BuffersNirmalya Chatterjee100% (1)
- 5: PH Measurement and Its Applications (Experiment) : ObjectivesDocument19 pages5: PH Measurement and Its Applications (Experiment) : ObjectivesNajmi NasirNo ratings yet
- Preparation of Buffers: An Experiment in pH ControlDocument6 pagesPreparation of Buffers: An Experiment in pH ControlGladys CastilloNo ratings yet
- 1Document8 pages1Isma WantiNo ratings yet
- Chem 131A: PH and Buffers ExperimentDocument4 pagesChem 131A: PH and Buffers ExperimentLinh NguyễnNo ratings yet
- 62 Experiment #5. Titration of An Acid Using A PH MeterDocument7 pages62 Experiment #5. Titration of An Acid Using A PH MeteryumnatehreemNo ratings yet
- 8 - Lab8-Potentiometric Titration of Acid MixtureDocument6 pages8 - Lab8-Potentiometric Titration of Acid MixtureHoang Huong TraNo ratings yet
- Sample Chemistry Undergraduate Laboratory ReportDocument14 pagesSample Chemistry Undergraduate Laboratory ReportApril TapayanNo ratings yet
- Designing a Buffer of a Specific pHDocument7 pagesDesigning a Buffer of a Specific pHZiana ManzarNo ratings yet
- Titration of The Weak Acid Potassium Hydrogen Phthalate (KHP)Document4 pagesTitration of The Weak Acid Potassium Hydrogen Phthalate (KHP)Kunwar Sanjeev SinghNo ratings yet
- LSM1101 Practical 1Document6 pagesLSM1101 Practical 1givena2ndchance100% (1)
- Acid Base TitrationDocument4 pagesAcid Base TitrationNeeta PandeyNo ratings yet
- CH142Exp5Titration PDFDocument7 pagesCH142Exp5Titration PDFSako RasheedNo ratings yet
- Nickel Ion analysisDocument6 pagesNickel Ion analysisShenella WilliamsNo ratings yet
- Determine Phosphoric Acid Content in Soft DrinksDocument4 pagesDetermine Phosphoric Acid Content in Soft DrinksNaveen KumarNo ratings yet
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDocument5 pagesAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- Ka & Molar Mass of a Weak AcidDocument7 pagesKa & Molar Mass of a Weak AcidLeslie Sarah100% (1)
- Online Titration LabDocument5 pagesOnline Titration LabMegan SarahNo ratings yet
- Quantitative Determination of Potassium Acid Phthalate KHPDocument17 pagesQuantitative Determination of Potassium Acid Phthalate KHPMichelle Cruz AbrilNo ratings yet
- Determining Ksp of Ca(OH)2Document4 pagesDetermining Ksp of Ca(OH)2Madel Tutor ChaturvediNo ratings yet
- UV/Vis Spectrophotometry and Fractional DistillationDocument9 pagesUV/Vis Spectrophotometry and Fractional DistillationAldayne ParkesNo ratings yet
- Experiment 1&2Document8 pagesExperiment 1&2Fatima AhmedNo ratings yet
- Experiment 8: Gas Chromatography (GC)Document4 pagesExperiment 8: Gas Chromatography (GC)Sergio BritanicoNo ratings yet
- Identification of An Unknown Amino AcidDocument7 pagesIdentification of An Unknown Amino AcidVanandiNo ratings yet
- Determine pH of samples using a pH meterDocument5 pagesDetermine pH of samples using a pH meterAjuba AbujaNo ratings yet
- Lab Format:: Lab 2: Determination of Carbonate/BicarbonateDocument5 pagesLab Format:: Lab 2: Determination of Carbonate/BicarbonateAnaya FatimaNo ratings yet
- Phytochemical Analysis Laboratory Manual: Hebron University Prepared by Dr. Abdel Qader A. QawasmehDocument20 pagesPhytochemical Analysis Laboratory Manual: Hebron University Prepared by Dr. Abdel Qader A. QawasmehQOSSAY ALHROUSHNo ratings yet
- Eq WT of Salicyclic AcidDocument4 pagesEq WT of Salicyclic AcidRaj Bhan100% (1)
- Acids & Bases, Titrations & BuffersDocument6 pagesAcids & Bases, Titrations & BuffersAhmed KurdishNo ratings yet
- Practical Handbook of Pharmaceutical Chemistry for M.PharmFrom EverandPractical Handbook of Pharmaceutical Chemistry for M.PharmNo ratings yet
- Practical Manual of Analytical ChemistryFrom EverandPractical Manual of Analytical ChemistryRating: 4.5 out of 5 stars4.5/5 (3)
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Chapter 15 Slides (Modified) - Student Version PDFDocument85 pagesChapter 15 Slides (Modified) - Student Version PDFRoberto BeltNo ratings yet
- Chem 163 Exam #1 Study GuideDocument3 pagesChem 163 Exam #1 Study GuideRoberto BeltNo ratings yet
- Chapter 14 Slides - Student VersionDocument52 pagesChapter 14 Slides - Student VersionRoberto BeltNo ratings yet
- Fall 2020 CHE 441 - Process Controls Laplace TableDocument1 pageFall 2020 CHE 441 - Process Controls Laplace TableRoberto BeltNo ratings yet
- Section 5.4 Shell-And-tube Heat Exchanger - CorrectedDocument17 pagesSection 5.4 Shell-And-tube Heat Exchanger - CorrectedPerumalSamyNo ratings yet
- Student LectureDocument1 pageStudent LectureRoberto BeltNo ratings yet
- Thermal Conductivity Prandtl NumberDocument2 pagesThermal Conductivity Prandtl NumberRoberto BeltNo ratings yet
- Results Change in Egg Weight (G) in Solution Over Time (Mins.)Document2 pagesResults Change in Egg Weight (G) in Solution Over Time (Mins.)Roberto BeltNo ratings yet
- HW #3 Chem 331 Assigned Friday Sept 13, Due Friday Sept 27 CH 17: 9, 13, 16, 34, 41, 43 CH 18: 8, 13, 17, 21, 38 CH 19: 7, 8, 12Document1 pageHW #3 Chem 331 Assigned Friday Sept 13, Due Friday Sept 27 CH 17: 9, 13, 16, 34, 41, 43 CH 18: 8, 13, 17, 21, 38 CH 19: 7, 8, 12Roberto BeltNo ratings yet
- Organic Materials in The Wall Paintings in Pompei: A Case Study of Insula Del CentenarioDocument10 pagesOrganic Materials in The Wall Paintings in Pompei: A Case Study of Insula Del CentenariopostiraNo ratings yet
- 2305 Gulf DieselectDocument1 page2305 Gulf DieselectEltjon Pumi100% (1)
- TMA Guide for Secondary StudentsDocument62 pagesTMA Guide for Secondary StudentsRevanth ShankarNo ratings yet
- Lecture 2 - HomeostasisDocument27 pagesLecture 2 - HomeostasisAndre Luis CostaNo ratings yet
- Gracilaria Handbook Culture PDFDocument49 pagesGracilaria Handbook Culture PDFFaizatulAsmaJaidinNo ratings yet
- Technical CatalogueDocument80 pagesTechnical CatalogueHermanNo ratings yet
- A1 Port Pier Condition SurveyDocument10 pagesA1 Port Pier Condition SurveyYuliar Azmi AdhitamaNo ratings yet
- Refineria de Cartagena (Reficar) Refinery Expansion - Hydrocarbons TechnologyDocument3 pagesRefineria de Cartagena (Reficar) Refinery Expansion - Hydrocarbons TechnologyGjorgeluisNo ratings yet
- FICHA TECNlCA ULEXITA CALCINADADocument2 pagesFICHA TECNlCA ULEXITA CALCINADATinxoLandivarNo ratings yet
- Nutrilite Vs PediasureDocument3 pagesNutrilite Vs Pediasuresuganthiaravind100% (3)
- Repair Instructions Powerspeed AttachmentDocument2 pagesRepair Instructions Powerspeed AttachmentGMNo ratings yet
- Basic Concepts of Superconductivity-1Document9 pagesBasic Concepts of Superconductivity-1Srikanth BatnaNo ratings yet
- SCIENCE 8 Q3 - WEEK 3 - LAS 1 Phase ChangeDocument2 pagesSCIENCE 8 Q3 - WEEK 3 - LAS 1 Phase ChangeGlin BarrientosNo ratings yet
- Chemistry Investigatory Project Content of Cold Drinks Available in The MarketDocument24 pagesChemistry Investigatory Project Content of Cold Drinks Available in The MarketSarojNo ratings yet
- The LNG Process ChainDocument8 pagesThe LNG Process ChainGHULAM MOHYUDDINNo ratings yet
- Essential Words For The TOEFLDocument28 pagesEssential Words For The TOEFLpritam goleNo ratings yet
- Sigma M-460-tcm67-8533Document4 pagesSigma M-460-tcm67-8533dambriellyNo ratings yet
- Degassing Aluminum Alloys Removes HydrogenDocument10 pagesDegassing Aluminum Alloys Removes Hydrogenlamia97No ratings yet
- NEBOSH GC3 Observations Sheet SampleDocument12 pagesNEBOSH GC3 Observations Sheet SampleShezi Bhatti100% (3)
- Multi-stage compression power calculationsDocument8 pagesMulti-stage compression power calculationsallovid0% (1)
- Caolin C. Britex 95 Qpros PDFDocument3 pagesCaolin C. Britex 95 Qpros PDFEduardo Pérez100% (1)
- Triple Effect EvaporatorDocument9 pagesTriple Effect Evaporatorjnmanivannan100% (1)
- Alkylation Unit: Capacity Installed and Available Technologies CatalystsDocument10 pagesAlkylation Unit: Capacity Installed and Available Technologies CatalystsMohammed AliraqiNo ratings yet
- Plastics in Medical Devices - 10Document4 pagesPlastics in Medical Devices - 10Emilio HipolaNo ratings yet
- General Specifi Cations: ADMAG TI Series AXG Magnetic FlowmeterDocument74 pagesGeneral Specifi Cations: ADMAG TI Series AXG Magnetic FlowmeterSteve WanNo ratings yet
- Effectiveness of Guava 1Document16 pagesEffectiveness of Guava 1Arellano Reyson100% (1)
- Jjmie: Synthesis and Characterization of Aluminum Composites Materials Reinforced With Tic Nano-ParticlesDocument8 pagesJjmie: Synthesis and Characterization of Aluminum Composites Materials Reinforced With Tic Nano-ParticlesMallappa KomarNo ratings yet
- Materials Found at Home: Properties, Uses, and SafetyDocument2 pagesMaterials Found at Home: Properties, Uses, and SafetyprecillaugartehalagoNo ratings yet
- EDTA Titration of Calcium and Magnesium IonsDocument3 pagesEDTA Titration of Calcium and Magnesium IonsAnonymous NxpnI6jC67% (3)
- Artcles AP Kharif 05Document33 pagesArtcles AP Kharif 05api-3833893No ratings yet