0% found this document useful (0 votes)
137 views6 pagesValidation Details PDF
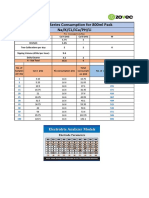
This document provides validation details for a CRP assay including reference range determination from normal samples, intra-assay and inter-assay precision studies, linearity, interlab correlation, and accuracy checks using manufacturer controls. Validation shows good agreement with expected results and reference ranges.
Uploaded by
BrijeshCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
137 views6 pagesValidation Details PDF
This document provides validation details for a CRP assay including reference range determination from normal samples, intra-assay and inter-assay precision studies, linearity, interlab correlation, and accuracy checks using manufacturer controls. Validation shows good agreement with expected results and reference ranges.
Uploaded by
BrijeshCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd