Professional Documents
Culture Documents
Seminar: Epidemiology
Uploaded by
Prasasti 19Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Seminar: Epidemiology
Uploaded by
Prasasti 19Copyright:
Available Formats
Seminar
Idiopathic pulmonary fibrosis
Luca Richeldi, Harold R Collard, Mark G Jones
Idiopathic pulmonary fibrosis is a prototype of chronic, progressive, and fibrotic lung disease. Healthy tissue is Published Online
replaced by altered extracellular matrix and alveolar architecture is destroyed, which leads to decreased lung March 29, 2017
http://dx.doi.org/10.1016/
compliance, disrupted gas exchange, and ultimately respiratory failure and death. In less than a decade,
S0140-6736(17)30866-8
understanding of the pathogenesis and management of this disease has been transformed, and two disease-modifying
Unità Operativa Complessa di
therapies have been approved, worldwide. In this Seminar, we summarise the presentation, pathophysiology, Pneumologia, Università
diagnosis, and treatment options available for patients with idiopathic pulmonary fibrosis. This disease has Cattolica del Sacro Cuore,
improved understanding of the mechanisms of lung fibrosis, and offers hope that similar approaches will transform Fondazione Policlinico
A. Gemelli, Rome, Italy
the management of patients with other progressive fibrotic lung diseases.
(Prof L Richeldi MD); National
Institute for Health Research
Epidemiology and wood dusts, agriculture and farming, viruses, and Southampton Respiratory
Idiopathic pulmonary fibrosis is the most common type of stone and silica.4,11,12 Biomedical Research Unit and
Clinical and Experimental
idiopathic interstitial pneumonia. Although the disease
Sciences, University of
has been considered rare, it occurs with similar frequency Genetic factors Southampton, Southampton,
to that of stomach, brain, and testicular cancers.1,2 Increasing evidence indicates that genetic susceptibility UK (Prof L Richeldi,
Incidence of idiopathic pulmonary fibrosis has risen over plays a part in the development of idiopathic pulmonary M G Jones PhD); and
Department of Medicine,
time, and in Europe and North America is estimated to fibrosis. Studies4,13–19 of familial interstitial
University of California,
range between 2·8 and 18 cases per 100 000 people pneumonia—ie, cases affecting two or more members of San Francisco, San Francisco,
per year.2,3 Little data are available for worldwide variation, the same biological family—have identified rare genetic CA, USA (H R Collard MD)
but incidence might be lower in Asia and South America, variants, including genes associated with surfactant Correspondence to:
where it is estimated to range from 0·5 to 4·2 cases per dysfunction (SFTPC, SFTPA2) and telomere biology Prof Luca Richeldi, Unità
Operativa Complessa di
100 000 individuals per year. (TERT, TERC, PARN, RTEL). Genome-wide association
Pneumologia, Università
Idiopathic pulmonary fibrosis is more common in men studies20–22 have identified common genetic variants, Cattolica del Sacro Cuore,
and is rare in people younger than 50 years (median age which account for about a third of the risk of disease Fondazione Policlinico
at diagnosis is about 65 years).4–6 Although disease course development. Although these studies do not indicate a A. Gemelli, Rome 00168, Italy
luca.richeldi@unicatt.it
is variable and somewhat unpredictable, the median direct causal link, the potential importance of alterations
survival time from diagnosis is 2–4 years.7 in host defence (MUC5B, ATP11A, TOLLIP), telomere
maintenance (TERT, TERC, OBFC1), and epithelial
Pathophysiology barrier function (DSP, DPP9) was identified.
Historically, idiopathic pulmonary fibrosis was considered A common gain-of-function variant in the gene
a chronic inflammatory disorder, which gradually MUC5B promoter region is the risk variant with the
progressed to established fibrosis. However, at the turn of largest genetic effect on development of both familial
the century, following the recognition that anti- and sporadic idiopathic pulmonary fibrosis (odds ratio
inflammatory therapy did not improve outcome, this 4–8 per allele).20,21,23–27 The MUC5B variant has low
concept was reassessed and, subsequently, an penetrance and in isolation does not seem to be causative
immunosuppressive therapeutic strategy incorporating of idiopathic pulmonary fibrosis. MUC5B encodes a
prednisolone and azathioprine was shown to increase mucin-5B precursor protein that contributes to airway
mortality.8,9 Idiopathic pulmonary fibrosis is now mucous production and might have an important role in
generally regarded as a consequence of multiple lung host defense.28,29 The site of altered MUC5B
interacting genetic and environmental risk factors, production has been localised to bronchiolar epithelium,
with repetitive local micro-injuries to ageing alveolar where it is proposed to increase protein concentrations
epithelium playing a central role. These micro-injuries that might either enhance injury as a result of reduced
initiate aberrant epithelial–fibroblast communication, the mucociliary clearance or impede normal lung repair.22,30
induction of matrix-producing myofibroblasts, and
considerable extracellular matrix accumulation and
remodelling of lung interstitium (figure 1). Search strategy and selection criteria
We searched PubMed for reports published in English between
Environmental exposures Jan 1, 1996, and Oct 1, 2016, using the search terms “pulmonary
Particulate inhalation is implicated in the pathogenesis fibrosis”, “fibrosing alveolitis”, “usual interstitial pneumonia”,
and progression of idiopathic pulmonary fibrosis. A and “nonspecific interstitial pneumonia”. We mostly selected
history of cigarette smoking is associated with publications from the past 5 years, although we also included
idiopathic pulmonary fibrosis development in most highly regarded older publications. Reviews are cited to provide
patients.10 However, multiple other environmental the reader with additional detail and references.
exposures have also been associated, including metal
www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8 1
Seminar
Dysfunctional epithelium Fibrogenesis Fibrosis telomeres is observed, and shortening of AEC2 telomeres
led to lung remodelling and fibrosis in a mouse model.34–36
A 2016 study37 showed that AEC2s from idiopathic
pulmonary fibrosis tissue have impaired renewal capacity,
consistent with AEC2 stem-cell failure. Abnormal behaviour
of alveolar epithelial cells is associated with epithelial
recapitulation of developmental pathways, including
Wnt/b-catenin and Sonic hedgehog pathways.38,39 Activated
• Genetic susceptibility • Epithelial cell apoptosis and • Extracellular matrix expansion alveolar epithelial cells secrete numerous fibrogenic growth
• Ageing senescence • Altered extracellular matrix
• Recurrent microinjury composition
factors and cytokines, including transforming growth
• Altered extracellular matrix factor β (TGF β) and platelet-derived growth factor, with
biomechanics aberrant epithelial mesenchymal cross-talk driving the
• Deficient fibroblast apoptosis
Fibroblast activation and • Alveolar collapse recruitment and activation of highly synthetic and
proliferation contractile myofibroblasts.40 These activated myofibroblasts
deposit an increased amount of altered extracellular matrix
components, which destroys normal alveolar architecture
and disrupts gas exchange. Multiple sources of
myofibroblasts are proposed, including resident
mesenchymal cell proliferation, lung interstitium pericytes,
Fibroblast recruitment circulating fibrocytes, epithelial mesenchymal transition,
and endothelial mesenchymal transition.33,41
Aberrant remodelling Progression from a normal to an abnormal extracellular
matrix in idiopathic pulmonary fibrosis is poorly
• Activation of epithelial cells • Alveolar stem-cell exhaustion understood, although evidence suggests that abnormal
• Basement membrane disruption • Basal cell dysfunction
• Dysregulated signalling • Abnormal extracellular matrix extracellular matrix deposition contributes to disease
• Immune activation remodelling pathogenesis.42,43 Changes in extracellular matrix
• Bronchiolisation
• Honeycomb cyst formation
composition alter cell behaviour considerably, and a
positive feedback loop between fibroblasts and aberrant
extracellular matrix promotes fibrosis.44 Both altered
Figure 1: Proposed mechanisms contributing to the pathogenesis of idiopathic pulmonary fibrosis extracellular matrix composition and stiffness might
Repetitive microinjuries to ageing alveolar epithelium activates alveolar epithelial cells to secrete multiple contribute to this process. The precise mechanisms by
fibrogenic growth factors, cytokines, and coagulants. This secretion leads to myofibroblast recruitment and which extracellular matrix stiffness is transduced by
activation from multiple sources, including resident mesenchymal cell proliferation, pericytes of the lung
interstitium, circulating fibrocytes, epithelial mesenchymal transition, and endothelial mesenchymal transition.
fibroblasts remains unclear, but integrins, the
These myofibroblasts deposit increased and altered extracellular matrix, with altered biomechanical stiffness, predominant receptors for cell adhesion to extracellular
which further contributes to myofibroblast activation in a positive feedback loop. Dysregulated repair of this matrix proteins, have a central role, with
injured lung parenchyma with abnormal activation of developmental pathways and bronchiolisation of the lung mechanosensitive protein–protein interactions occurring
occurs in parallel (histopathological images magnification ×250).
within adhesion complexes.45,46 Downstream of these
interactions, cell force-mediated activation of latent TGF
Intriguingly, patients with idiopathic pulmonary fibrosis β, and intrinsic mechanotransduction via the Rho–Rho
with the MUC5B gain-of-function variant might have a kinase pathway, promotes myofibroblast differentation.47–49
higher rate of survival than those without this variant.31 In parallel with abnormal extracellular matrix
This finding requires external validation, although in UK production is the occurrence of aberrant lung remodelling
patients with idiopathic pulmonary fibrosis, this variant with so-called bronchiolisation of alveolar tissue. At sites
was associated with a slower decline in lung function.26 of damaged alveolar epithelium, a regenerative response
associated with activation of the developmental pathway
Maladaptive repair process occurs.50 Abnormal activation of airway basal cells, which
Identification of pathological mechanisms of fibrogenesis reside in the conducting airways down to the respiratory
in idiopathic pulmonary fibrosis has been challenging; bronchioles and function as stem cells, is identified and
however, chronic dysregulation of type 2 alveolar epithelial might contribute to re-epithelisation of damaged alveolar
cells (AEC2s) is thought to be central. AEC2s are stem epithelium and resulting bronchiolisation of alveolar
cells within the lung that contribute to renewal of type 1 spaces.51–54 In a subset of patients with idiopathic
alveolar epithelial cells (AEC1s) during homoeostasis or pulmonary fibrosis, higher cilium gene expression was
after lung injury.32,33 Loss of AEC1s and abnormal AEC2s associated with increased microscopic honeycombing
are identified in idiopathic pulmonary fibrosis tissue, with (characterised by well defined walls), although whether
fibroblastic foci typically located adjacent to hyperplastic the bronchiolar abnormalities arise from de-novo
or apoptotic alveolar epithelial cells.8 In idiopathic bronchiolisation or from adjacent normal bronchiolar
pulmonary fibrosis tissue, premature shortening of AEC2 structures remains uncertain.55
2 www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8
Seminar
Clinical presentations, signs, and symptoms
Patients typically present with non-specific symptoms of Genetic predisposition
exertional dyspnoea with or without dry cough (figure 2).
This presentation might initially be attributed to ageing, Smoking, occupational dust
exposure, and other risk factors
deconditioning, or other comorbidities (eg, smoking history,
emphysema, cardiovascular disease, or obesity); therefore, Subclinical disease
(Velcro-type crackles)
clinical suspicion of idiopathic pulmonary fibrosis by
primary care physicians is required to prevent diagnostic
Onset of symptoms
delays. Occasionally, patients will present acutely, with days
to weeks of respiratory worsening, often accompanied by
Diagnosis (typically age 60–70 years)
fever and influenza-like symptoms. These acute
exacerbations require careful diagnostic distinction from
other forms of acute interstitial lung disease. Disease-modifying therapy
On physical examination, fine, high-pitched bibasilar
inspiratory crackles are usually heard (audio) and digital Comprehensive management
clubbing is present in roughly 30% of patients.56 Careful
attention to signs of connective tissue disease is essential
to rule out associated diseases. In established disease, Figure 2: Conceptual model of idiopathic pulmonary fibrosis across an individual’s life course
pulmonary function tests identify restrictive disease
(reduced total lung capacity) and abnormal gas exchange
(reduced capacity for carbon monoxide diffusion).4 Early A B
disease, or disease coexisting with emphysema that
pseudonormalises volumes, might demonstrate normal
spirometry and plethysmography, with only an isolated
reduction in diffusion.
Diagnosis
Idiopathic pulmonary fibrosis is diagnosed by identification
of a pattern of usual interstitial pneumonia on the basis of
radiological or histological criteria in patients without
evidence of an alternative cause.4,57,58 This approach is
endorsed in consensus guidelines worldwide and
has helped to standardise idiopathic pulmonary fibrosis C D
diagnosis. A major challenge to clinicians is exclusion of
other idiopathic interstitial pneumonias and of known
causes of interstitial lung disease, such as domestic and
occupational exposures, connective tissue disease, and
drug toxicity. Such exclusion is of particular importance
because the usual interstitial pneumonia pattern is not
exclusive to idiopathic pulmonary fibrosis and might also
be associated with other conditions, including chronic
hypersensitivity pneumonitis, asbestosis, connective tissue
diseases, and drug toxicity. Many patients have histories of
environmental exposures, medications, and symptoms
that require clinicians to make judgments regarding the Figure 3: Radiological and histological patterns of usual interstitial pneumonia
relevance of the cause of disease. Coronal reconstruction of high-resolution CT of the chest, showing the basal predominance of subpleural
High-resolution CT of the chest enables detailed honeycombing, which is typical of a usual interstitial pneumonia pattern. High-resolution chest CT axial image
assessment of lung parenchyma and has revolutionised taken at the level of the lower lobes showing multilayered subpleural honeycombing without evidence of features
inconsistent with a pattern of usual interstitial pneumonia (B). Histological pattern of usual interstitial pneumonia
the investigation of suspected idiopathic pulmonary at low power (magnification ×100). Typical spatial and temporal heterogeneity can be observed with subpleural
fibrosis. Reticular opacities, associated with traction fibrosis and microscopic honeycombing, less-fibrotic central lung tissue, and fibroblast foci (C). A fibroblast focus is
bronchiectasis and clusters of subpleural, cystic airspaces shown at the interface between fibrotic and less-involved lung tissue at higher power (magnification ×400).
of similar diameters (typically 3–10 mm) with The asterisk indicates the fibroblast focus (D).
honeycombing, in a predominantly bilateral, peripheral,
and basal distribution are typical of the usual interstitial Patients with reticular abnormality in a subpleural, basal See Online for Audio
pneumonia pattern (figure 3; video), whereas features predominance, but without honeycombing, are See Online for Video
such as mosaic attenuation, ground-glass abnormality, considered to have a possible usual interstitial
and nodules suggest an alternative diagnosis.4,59–61 pneumonia pattern.
www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8 3
Seminar
When high-resolution CT features are non-diagnostic, fibrosis diagnosis with varying degrees of diagnostic
surgical lung biopsy is advised. In-hospital mortality certainty. A dynamic multidisciplinary discussion between
after elective biopsy in patients younger than 65 years physicians, radiologists, and pathologists experienced in
and with few comorbidities is less than 2%, but every diagnosis of interstitial lung disease increases diagnostic
patient still requires careful consideration as to whether agreement, and thus is considered the diagnostic gold
the risks of surgical lung biopsy outweigh the potential standard, although a few patients remain unclassifiable.67,68
benefits of diagnostic information.62 In older patients, Accurate prognostication is difficult because the
those with comorbidities (updated Charlson score >1), natural history of idiopathic pulmonary fibrosis is highly
clinically significant physiological impairment, or for variable. Some patients progress rapidly, others quite
non-elective procedures, the risk is greater and surgical slowly, and others have sudden worsening after periods
lung biopsy should generally be avoided. When of stability.4 Shortened survival time is associated with
considering undertaking surgical lung biopsy, the pretest factors including older age, severe physiological
probability of a subsequent histopathological diagnosis impairment, low body-mass index, greater radiological
of usual interstitial pneumonia in patients with possible disease extent, and presence of pulmonary hypertension,
usual interstitial pneumonia on high-resolution CT is emphysema, and bronchogenic cancer.7
increased with older age, male sex, and the presence of To predict individual patient prognosis, risk models
traction bronchiectasis.63,64 that incorporate demographic, clinical, and physiological
Histopathological findings show that the usual variables have been developed, including the du Bois
interstitial pneumonia pattern is characterised by model69 and the gender, age, physiology index.70,71 This
interstitial fibrosis with spatial heterogeneity and patchy index70 incorporates gender, age, and lung physiology
involvement of lung parenchyma, areas of marked fibrosis, variables to identify three disease stages with a 1 year
architectural distortion, and microscopic honeycombing mortality risk of 6%, 16%, and 39% respectively. The
(cystic airspaces lined by bronchiolar epithelium, typically calculation of such an index at diagnosis might aid the
filled with mucin; figure 3).4 At the interface between clinician to refine prognosis, help to guide management
fibrotic and normal lung tissue are aggregates of decisions, such as lung transplantation timing, and allow
proliferating fibroblasts and myofibroblasts—fibroblastic appropriate life planning.
foci—within a myxoid-appearing matrix. Fibroblast foci
are a key histopathological feature of usual interstitial Clinical genetic testing
pneumonia pattern, which represent areas of active In individuals with a family history of interstitial lung
disease, and their absence excludes a definite disease suggestive of familial interstitial pneumonia,
histopathological usual interstitial pneumonia diagnosis. genetic testing might be appropriate after counselling
In 2D fibroblastic foci are considered small, distinct because it can aid disease course prognostication and
lesions; however, in 3D the foci form heterogeneous lung transplant risk stratification. Familial interstitial
structures with large variations in shape and volume.65,66 pneumonia transmission is thought to be autosomal
Clinical features, imaging, and histopathology all play dominant with reduced penetrance; however, disease
important roles in the diagnosis of idiopathic pulmonary penetrance of individual disease-associated genes
fibrosis. A patient might receive an idiopathic pulmonary remains unclear and so family member risk might be
difficult to quantify.72,73
Genetic testing in sporadic idiopathic pulmonary
fibrosis is not recommended unless patients have a
ion
personal or family history of extrapulmonary features
progress
Disease associated with a telomeropathy, such as aplastic
• End-of-life anaemia, cryptogenic cirrhosis, or premature greying.74 If
preferences
• Oxygen assessment • Lung transplantation such a history is identified, clinical peripheral blood
• Pulmonary • Palliative care mononuclear cell telomere length testing might be
• Disease-modifying rehabilitation • Supplemental
therapy
oxygen
considered, and if telomere length is short (<10% for age),
• Clinical trials +
• Patient education
investigation for telomerase-related gene mutations
+
• Smoking cessation should be done.73
• Treatment of
Advanced disease
comorbidities
• Vaccination Clinical management
Prompt referral of patients with known or suspected
Early disease
idiopathic pulmonary fibrosis to a centre with expertise
in idiopathic pulmonary fibrosis care is advised, because
Disease-modifying treatment vs Symptom control
delayed access is independently associated with
increased risk of death.75 Referral provides patients with
Figure 4: A step-by-step approach to the comprehensive management of access to expertise in diagnosis and management,
patients with idiopathic pulmonary fibrosis including initiation of disease-modifying therapy,
4 www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8
Seminar
monitoring, side-effect control, and non-pharmacological
support (figure 4).4,76 In addition to idiopathic pulmonary Panel: Therapies identified in clinical trials as harmful,
fibrosis focused management, comorbidities commonly ineffective, or effective in the treatment of idiopathic
associated with idiopathic pulmonary fibrosis might be pulmonary fibrosis
present, including emphysema, pulmonary Potentially harmful therapies
hypertension, gastro-oesophageal reflux disease, and • Ambrisentan81
obstructive sleep apnoea.77–80 • Everolimus82
• Prednisolone, azathioprine, acetylcysteine9
Disease-modifying therapy • Warfarin83
Standardisation of idiopathic pulmonary fibrosis
diagnostic criteria has enabled large, multicentre, Potentially ineffective therapies
randomised placebo-controlled trials of proposed disease- • Bosentan84
modifying drugs. Randomised controlled trials identified • Imatinib85
that various putative therapies (eg, prednisolone and • Macitentan86
azathioprine, acetylcysteine, and warfarin) were • Acetylcysteine87
ineffective or harmful; a landmark contribution to • Sildenafil88
idiopathic pulmonary fibrosis patient care (panel).9,83 Effective disease-modifying therapies
Through these randomised controlled trials, two large • Nintedanib89
phase 3 development programmes identified the first • Pirfenidone90,91
effective disease-modifying therapies for idiopathic
pulmonary fibrosis—nintedanib and pirfenidone. Both
drugs are now approved worldwide for idiopathic 2004. The first study98 reported a significant
pulmonary fibrosis treatment, which has transformed reduction (56·3%) in the relative decline in vital
patient management.89,90 capacity in patients treated with pirfenidone. Two
Nintedanib is a tyrosine kinase inhibitor that subsequent phase 3 trials91 compared pirfenidone with
suppresses multiple signalling receptors implicated in placebo over 72 weeks. The effect of pirfenidone on
fibrosis pathogenesis, including fibroblast growth factor forced vital capacity decline was discordant in these
receptor, platelet-derived growth factor receptor, and two trials; one trial confirmed the initial phase 3 results
vascular endothelial growth factor receptor.92,93 A phase 2 whereas the other trial found no significant difference
study of nintedanib in idiopathic pulmonary fibrosis between the groups. A fourth phase 3 study90 was done
identified a dose-dependent trend towards reduced lung over 52 weeks in patients with a forced vital capacity of
function decline and reduction in acute exacerbation 50–90% predicted and diffusion capacity for carbon
incidence.94 Two subsequent phase 3 trials were done monoxide of 30–90% predicted. The relative decline in
comparing nintedanib with a placebo.89 Inclusion forced vital capacity was significantly less in the active
required a forced vital capacity of greater than or equal treatment group (54·9%) compared with the placebo
to 50% of the predicted value and a diffusion capacity of group. Pooled analyses90,99 of 1247 patients from phase 3
the lung for carbon monoxide of between 30% and trials reported that fewer patients died in the
79% predicted. Over 52 weeks, relative decline in forced pirfenidone groups than in the placebo groups
vital capacity was significantly less in the active treatment (HR 0·52, 95% CI 0·31–0·87; p=0·01) and that fewer
group than in the control group in both trials patients had a decrease in 6 min walk distance in the
(47·9% and 55·1%).89 In the INPULSIS-2 trial,89 a pirfenidone groups than in the placebo groups.
significant difference in time to first acute exacerbation Nintedanib and pirfenidone have a similar effect on
was identified. A prespecified sensitivity analysis based rate of decline in forced vital capacity over 1 year
on pooled data from both trials of confirmed or suspected (figure 5).89–91 Neither drug has prospectively shown a
acute exacerbations adjudicated centrally by investigators survival benefit in these trials, although both show a
masked to treatment group showed a benefit of trend in favour of a reduction in mortality. Therefore,
nintedanib (hazard ratio [HR] 0·32, 95% CI 0·16–0·65; the safety profile and tolerability of these drugs will
p=0·001).89 Post-hoc analyses, which categorised patients influence patient and provider choice. Nintedanib and
by age, smoking history, and forced vital capacity, showed pirfenidone have good safety profiles within clinical
a consistent effect of nintedanib across subgroups.95 trials, with acceptable tolerability in most patients,
Pirfenidone is an orally administered pyridine although roughly a fifth of patients might discontinue
with combined anti-inflammatory, antioxidant, and treatment because of side-effects or disease progression.
antifibrotic actions, although the precise mechanism For both drugs, the primary safety concern is
of action is unknown.96,97 Regulation of TGF β in vitro, transaminitis, and both drugs require regular
and inhibition of fibroblast and collagen synthesis has monitoring of liver function. Pirfenidone can cause
been shown in animal models of lung fibrosis.97 Four gastrointestinal (dyspepsia and anorexia) and
phase 3 trials of pirfenidone have been done since dermatological (photosensitivity) side-effects.100
www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8 5
Seminar
0 bleeding due to the antivascular endothelial growth
factor receptor activity of the drug.102 Side-effects are
Relative decline in forced vital capacity (%)
minimised with food, and in the case of nintedanib, with
loperamide. Persistent side-effects are typically responsive
to temporary dose reduction or cessation.
–50
Because idiopathic pulmonary fibrosis is a chronic,
invariably progressive disease most patients should
begin therapy with one of these two drugs at the time of
diagnosis, with exceptions for severe and, perhaps,
Disease-modifying therapy asymptomatic disease. This recommendation is based on
–100
Placebo the notion that the earlier irreversible destruction of lung
0 12 24 36 48 52 can be slowed, the more potential benefit there is to
Time (weeks) patients. The known effects of continued treatment on
Figure 5: Effect of disease-modifying therapy on lung function decline disease progression must be balanced against the safety
The disease-modifying therapies nintedanib and pirfenidone roughly half the and tolerability profile in individual patients to determine
relative rate of decline of forced vital capacity compared with placebo over the value of treatment.
52 weeks in patients with idiopathic pulmonary fibrosis.89–91 Over 52 weeks the
mean decline in forced vital capacity in an untreated patient with idiopathic
pulmonary fibrosis is about 200 mL. Lung transplantation
In selected patients with idiopathic pulmonary fibrosis,
lung transplantation can improve quality of life and
prolong survival, with a 5 year survival rate post-
B transplantation of about 50%.4,103 However, only a few
patients receive this intervention because of the medical
complexity of the surgery and post-surgical treatment,
and the restricted supply of donor organs. In view of the
A heterogeneity of the disease course of idiopathic
pulmonary fibrosis, the optimum timing of referral for
lung transplantation evaluation is unclear, but many
C patients are referred too late in the course of their
Acute disease. Therefore, lung transplantation should be
clinical
deterioration
discussed with individual patients early in their disease
course and referral for evaluation should be made if
objective evidence of disease progression exists.
Acute respiratory deterioration
Patients with idiopathic pulmonary fibrosis might have
acute respiratory deteriorations, with development of
new or worsening dyspnoea and increased oxygen
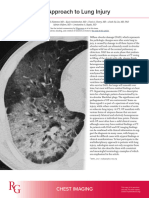
Figure 6: Radiological and histopathological changes that occur during an acute exacerbation of idiopathic requirements. These events are highly significant, with
pulmonary fibrosis median survival of only 3–4 months postevent. Acute
A patient with a diagnosis of idiopathic pulmonary fibrosis with an axial chest high-resolution CT image taken at respiratory deterioration can occur as a result of
the level of the carina demonstrating subpleural reticulation and areas of traction bronchiectasis (A). The patient is
admitted to hospital after the onset of worsening dyspnoea. An axial chest high-resolution CT image taken at the
numerous known causes (eg, infection); when idiopathic,
level of the carina in the same patient shows that the predominant pattern is a diffuse ground-glass abnormality such deterioration is commonly referred to as acute
becoming confluent with consolidation posteriorly (the so-called anterior-posterior density gradient), which is exacerbation of idiopathic pulmonary fibrosis.104 Acute
characteristic of an acute exacerbation of idiopathic pulmonary fibrosis (B). Minor respiratory motion artifact is exacerbation is thought to occur in roughly 5–15% of
observed because of the patient’s dyspnoea during image acquisition. Although not obvious on this image, a small
right pneumothorax is present and a chest drain has been positioned in the right pleural space. Surgical lung
patients with idiopathic pulmonary fibrosis annually and
biopsy has no routine diagnostic role in cases of suspected acute exacerbation in view of high non-elective is more common in patients with physiologically and
morbidity; when performed the histopathology slides identify that the alveolar septa are thickened by oedematous functionally advanced disease.105 By definition, acute
fibrosis and mild inflammation (C). The alveolar spaces show consolidation by fibrin and hyaline membranes, exacerbation is characterised by new bilateral diffuse
consistent with acute lung injury and diffuse alveolar damage (magnification ×400).
ground-glass opacities and consolidation on high-
resolution CT (figure 6). Histologically, diffuse alveolar
Nintedanib can cause gastrointestinal (diarrhoea and damage is superimposed on a usual interstitial
nausea) side-effects.101 Treatment with nintedanib in pneumonia pattern, but surgical lung biopsy should not
combination with full-dose anticoagulants or in patients generally be considered because of high non-elective
who have had a major bleeding event should be morbidity rates.62,106 A 2016 working group report105 on
considered if the anticipated benefit outweighs the acute exacerbation has suggested that the definition
potential risk, in view of the theoretical increased risk of should be broadened to include any acute respiratory
6 www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8
Seminar
deterioration with new widespread alveolar abnormality in which idiopathic pulmonary fibrosis was diagnosed
on high-resolution CT of the chest not fully explained by radiologically if either honeycomb lung destruction or
cardiac failure or fluid overload. traction bronchiectasis and a reticular abnormality
In patients with acute respiratory deterioration, consistent with fibrosis were present in a basal and
identification of any potentially treatable causes is a peripheral predominance.89,90 Post-hoc subgroup analysis114
priority. Extraparenchymal causes such as pulmonary of these patients showed that the rate of decline in forced
embolism, pneumothorax, and pleural effusion should vital capacity was identical in both groups.
be excluded. If safe to do, CT angiography with high- Multidisciplinary team discussion was endorsed by
resolution CT is the diagnostic test of choice. Infection guidelines as the gold standard for idiopathic pulmonary
should be suspected in consistent clinical cases and fibrosis diagnosis following the identification that
managed appropriately. In cases of acute exacerbation, substantial differences in the diagnosis might be
high-dose glucocorticoids are conditionally recommended reached by individuals working in isolation compared
by international guidelines; 4 however, no controlled trial with a dynamic face-to-face interaction between
data have demonstrated efficacy or safety of these drugs.107 clinicians, radiologists, and pathologists whereby
Guidelines make a conditional recommendation against interobserver agreement and diagnostic confidence is
mechanical ventilation in acute exacerbation, but this increased.67 Two studies115,116 identified that agreement
might be appropriate in selected cases, such as the between multidisciplinary teams in academic
bridging of a patient to lung transplantation. institutions is effective for a diagnosis of idiopathic
pulmonary fibrosis, with multidisciplinary teams
Symptom-focused therapy making the diagnosis of idiopathic pulmonary fibrosis
Adjunctive symptom-based management is important in with higher confidence and more frequently than
view of the high symptom burden of idiopathic pulmonary clinicians or radiologists independently. However, only
fibrosis, including dyspnoea and cough.108 In patients a small number of studies indicate how the
with chronic cough possible contributing comorbidities, multidisciplinary team approach is implemented in
such as gastro-oesophageal reflux disease, should be routine clinical care, and whether this affects diagnostic
considered. Opiates might reduce anxiety, dyspnoea, and accuracy and treatment decisions. To standardise
cough.109 Some evidence suggests that corticosteroids individual patient diagnosis increased understanding of
could be effective in the treatment of chronic cough.110 this process is required.
Symptoms are often refractory to standard pharma Interpretation of high-resolution CT scans has become
cological intervention. Pulmonary rehabilitation improves increasingly important in the diagnosis of idiopathic
dyspnoea and quality of life, and might improve function pulmonary fibrosis, and only a few patients now undergo
status.111–113 Education programmes, and patient support surgical lung biopsy. Transbronchial lung cryobiopsy
groups can help to minimise the effect of dyspnoea on with a flexible bronchoscope is an alternative method for
activities of daily living and to reduce the psychological sampling lung parenchyma proposed to have lower
burden of idiopathic pulmonary fibrosis. Supplemental complication and mortality rates, although large
oxygen therapy should be considered to treat hypoxaemia. multicentre prospective studies are required to confirm
With advancing disease, the involvement of palliative-care safety and diagnostic accuracy in idiopathic pulmonary
physicians and end-of-life planning should be discussed fibrosis before introducing it in clinical practice.117,118
in the outpatient setting.
Use of modifying therapies in patients outside of
Controversies and uncertainties clinical trials
Diagnosis of idiopathic pulmonary fibrosis The phase 3 studies of nintedanib and pirfenidone were
Approval of disease-modifying therapies for idiopathic designed to select a fairly homogenous population of
pulmonary fibrosis has increased the focus on early and patients with mild to moderate idiopathic pulmonary
accurate diagnosis with the aim of improving long-term fibrosis. Patients with severe functional impairment
treatment outcome. The diagnostic certainty of idiopathic (forced vital capacity <50% predicted or diffusion capacity
pulmonary fibrosis depends on the presence or absence of the lung for carbon monoxide <30% predicted) or
of specific morphological criteria; the approval of safe and major comorbidities, such as severe pulmonary
effective therapies provides a timely opportunity to review hypertension, were excluded. In many countries, use of
this approach because only patients with idiopathic nintedanib or pirfenidone is limited by drug regulatory
pulmonary fibrosis can receive these therapies.4 agency approval and reimbursement rules. Both drugs
Broadening of the radiological diagnostic criteria of received regulatory approval in the USA without any
idiopathic pulmonary fibrosis has gained considerable severity threshold.119 Although the tolerability of disease-
interest. The presence of honeycomb lung destruction is modifying therapy in patients with more severe
required for a definite radiological diagnosis of idiopathic physiological impairment might be reduced, in post-hoc
pulmonary fibrosis. Although this criterion was applied in analyses of phase 3 clinical trials no evidence suggests
pirfenidone trials, it was not used in nintedanib trials, that therapeutic efficacy varies with disease severity, and
www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8 7
Seminar
so informed discussion with individual patients outside Gastro-oesophageal reflux
of clinical trial settings would seem reasonable before Gastro-oesophageal reflux disease is common in patients
commencing treatment.95,120 with idiopathic pulmonary fibrosis, and chronic silent
microaspiration, as a source of repetitive lung injury, has
Identification and management of non-responding been proposed as a risk factor for development and
patients progression of idiopathic pulmonary fibrosis.124,125
Lung function will decline in patients with idiopathic However, no prospective data exist that support a causative
pulmonary fibrosis despite treatment; however, clinical or prognostic association. Treatment of gastro-oesophageal
assessment for disease progression and therapeutic reflux disease was associated with less radiological fibrosis
response is challenging. How to define failure to respond and was an independent predictor of increased survival
to treatment in an individual patient, and whether to time in a retrospective analysis126 of patients with idiopathic
consider cessation or alteration of therapy in patients with pulmonary fibrosis. Post-hoc analysis127 of three
objective disease progression, remains unclear. Rate of randomised controlled trials found that patients taking
change in forced vital capacity for an individual patient is antacid treatment had a smaller decrease in forced vital
variable over time; therefore, it is not possible to evaluate capacity than those not taking these therapies. By contrast,
therapeutic response by comparison of forced vital capacity post-hoc analysis128 of three phase 3 trials of pirfenidone in
trends preceding and following the commencement of idiopathic pulmonary fibrosis found that antacid therapy
disease-modifying therapy. Post-hoc analysis of patients did not improve outcomes and might be associated with
given pirfenidone shows that patients who have either a increased risk of infection in patients with a forced vital
10% or greater decline in forced vital capacity or hospital capacity of less than 70% predicted.
admission in the first 6 months of treatment still have a Non-acidic components of gastric acid are present in the
lower risk of forced vital capacity decline or death in the bronchoalveolar lavage fluid of patients with idiopathic
subsequent 6 months than do those given placebo.121 pulmonary fibrosis; as such, surgical management might
In the UK, the National Institute for Health and Care prove more effective than medical antacid therapy.129
Excellence recommends that nintedanib or pirfenidone Investigators of a single-centre retrospective trial129 of
be discontinued if after 12 months evidence shows laparoscopic antireflux surgery reported a decreased, albeit
disease progression (defined as a decrease in forced vital non-significant, decline in forced vital capacity. Prospective
capacity of ≥10% predicted); however, it is unclear studies of treatments for gastro-oesophageal reflux disease
whether this represents treatment failure.122 Because an are required for patients with idiopathic pulmonary fibrosis
individual’s disease course cannot be predicted, we do before the routine commencement of medical or surgical
not know what the rate of decline might have been therapies for asymptomatic reflux can be considered.
without treatment nor whether withdrawal of treatment
might provoke a precipitous decline. In patients with Microbiome and antimicrobial treatments
objective evidence of substantial physiological or Infection might influence fibrosis initiation and
radiological disease progression, a change of disease- progression.29,130 Whether alterations in the microbiome
modifying therapy might be considered, whereas in represent a causal factor or a determinant of disease
patients with advanced disease in whom the treatment behaviour possibly influenced by genetic polymorphisms
burden is affecting quality of life, discontinuation of such as MUC5B or TOLLIP, a surrogate finding of
treatment might be appropriate. another disease process such as microaspiration, or a
marker of lung structure derangement in idiopathic
Incorporation of genetics into routine clinical pulmonary fibrosis, remains uncertain.
management Studies into whether antimicrobials might influence
Considerable advances have been made in understanding the idiopathic pulmonary fibrosis disease course are
of the influence of genetics on the risk of developing ongoing. A phase 2 study of cotrimoxazole was done in
sporadic idiopathic pulmonary fibrosis and possibly patients with fibrotic idiopathic interstitial pneumonia
disease behaviour. Individuals with the MUC5B variant following the observation of a clinical improvement in
in the general population have increased prevalence of patients with advanced fibrotic lung disease treated with
subclinical interstitial lung abnormalities, and MUC5B is long-term oral cotrimoxazole.131,132 No difference was
most consistently associated with the risk of development identified in the primary endpoint of change in forced
of idiopathic pulmonary fibrosis.24 Genotype might also vital capacity over 12 months; however, a possible
influence response to pharmacological therapy, with a reduction in mortality was observed in patients who
For more on the EME-TIPAC trial 2015 post-hoc analysis123 showing that the TOLLIP adhered to treatment. An ongoing phase 3 study is
see https://www. genotype might determine a beneficial or harmful effect investigating the relevance of these findings.
clinicaltrialsregister.eu, number
2014-004058-32
of acetylcysteine therapy. The translation of these findings
to routine clinical practice will require robust prospective Biomarkers
studies to define the role for genetics in diagnosis and Biomarkers for diagnosis, prognosis, and response to
treatment of idiopathic pulmonary fibrosis. therapy prediction could help to address controversies
8 www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8
Seminar
regarding diagnosis and management of idiopathic response to disease-modifying therapy is uncertain.
pulmonary fibrosis. Although numerous potential Finally, although common pathogenic pathways of fibrosis
biomarkers have been studied, including genetic have been proposed, it is unclear whether antifibrotic
polymorphisms, gene expression profiles, CCL18, drugs with proven efficacy in idiopathic pulmonary
collagen neoepitopes, MMP7, and SPD, as yet, none is fibrosis will translate to other fibrotic diseases with few
prospectively validated for clinical practice.31,133–137 Major treatment options.140 The challenge of the next decade will
research efforts are ongoing towards biomarker be to address these questions while developing targeted
validation through collaborative multicentre, prospective therapies for use in combination with current treatments
cohorts with longitudinal data collection and biobanking. to halt fibrosis progression and maintain quality of life for
patients with idiopathic pulmonary fibrosis.
Combination therapies Contributors
Monotherapies only slow disease progression; therefore, All authors contributed to the design and writing of this manuscript, and
a clear priority is the development of approaches to halt, to the development of all figures.
or even reverse idiopathic pulmonary fibrosis. An initial Declaration of interests
step will be to study novel therapies combined with LR reports grants and personal fees from InterMune; and personal fees
from Biogen, Global Blood Therapeutics, Sanofi Aventis, Roche,
currently approved therapies. In a 2016 clinical trial,138 ImmuneWorks, Boehringer Ingelheim, Celgene, FibroGen, Promedior,
the addition of the glutathione precursor acetylcysteine Bayer, Asahi Kasei, and Pliant Therapeutics. HRC reports personal fees
to pirfenidone unexpectedly increased the rate of decline from Medimmune, Bayer, Boehringer Ingelheim, Xfibra, Genoa, Gilead,
in forced vital capacity, demonstrating the importance of Moerae Matrix, PharmAkea, Prometic, the Pulmonary Fibrosis
Foundation, aTyr Pharma, Global Blood Therapeutics, Veracyte, Patara,
randomised controlled trials. Studies of the drug–drug Alkermes, Takeda, Pharma Capital Partners, and Bristol-Myers Squibb,
interaction of pirfenidone and nintedanib are in outside the submitted work. MGJ declares no competing interests.
progress, although pharmacokinetic interactions Acknowledgments
between the drugs, in particular cumulative MGJ acknowledges support from the Wellcome Trust through a training
gastrointestinal effects, might preclude this, with some fellowship. We thank individuals who provided assistance with this
evidence of a lower exposure of nintedanib when added manuscript, including Simon Walsh (Kings College London, London,
UK) for the radiology images, film, and legends, Kirk Jones (University
to pirfenidone.139 of California, San Fransisco, San Francisco, CA, USA) for the
Clinical trial design in idiopathic pulmonary fibrosis histopathology images and legends, Anuchana Patise (Southampton
has been altered by the approval of nintedanib and National Institute for Health Research [NIHR] Biomedical Research
Unit, Southampton, UK) for assistance with figure preparation,
pirfenidone, which are now the standard of care.
Shandra Knight (National Jewish Health, Denver, CO, USA) for
Identification of drugs that are superior to nintedanib assistance with the literature search, and Giacomo Sgalla (Southampton
and pirfenidone will probably require innovative trial NIHR Biomedical Research Unit) for provision of the acoustic recording.
design, such as use of composite endpoints. As our We apologise to the authors whose work we could not cite because of
space constraints.
understanding of idiopathic pulmonary fibrosis
continues to increase, novel molecular classification References
1 International Agency for Research on Cancer. Cancer incidence in
approaches are likely to support the addition of targeted five continents. 2014. http://www.iarc.fr/en/publications/pdfs-
therapies to the pleiotropic mechanisms of action of the online/epi/sp164/CI5volX_Full.pdf. (accessed Oct 1, 2016).
approved drugs. 2 Hutchinson J, Fogarty A, Hubbard R, McKeever T. Global incidence
and mortality of idiopathic pulmonary fibrosis: a systematic review.
Eur Resp J 2015; 46: 795–806.
Conclusions and future directions 3 Hopkins RB, Fell C, Dion G, Kolb M. Epidemiology and survival of
In less than 10 years, the landscape of idiopathic pulmonary idiopathic pulmonary fibrosis from national data in Canada.
Eur Respir J 2016; 48: 187–95.
fibrosis has been transformed. Many no longer consider
4 Raghu G, Collard HR, Egan JJ, et al, for the ATS/ERS/JRS/ALAT
pulmonary fibrosis to be idiopathic, with interaction Committee on Idiopathic Pulmonary Fibrosis Study Group.
between causal factors, including genetic polymorphisms, An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary
fibrosis: evidence-based guidelines for diagnosis and management.
ageing, and environmental exposures, which culminate in Am J Respir Crit Care Med 2011; 183: 788–824.
a maladaptive repair process of injured lung. Advances in 5 American Thoracic Society. Idiopathic pulmonary fibrosis:
understanding of disease pathogenesis integrated with the diagnosis and treatment. International consensus statement.
establishment of methodologies to do large multicentre American Thoracic Society (ATS), and the European Respiratory
Society (ERS). Am J Respir Crit Care Med 2000; 161: 646–64.
randomised controlled trials have resulted in the approval 6 Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G.
of the first drugs to modify the disease course of idiopathic Incidence and prevalence of idiopathic pulmonary fibrosis.
pulmonary fibrosis. Am J Respir Crit Care Med 2006; 174: 810–16.
7 Ley B, Collard HR, King TE. Clinical course and prediction of survival
Despite these achievements, various problems remain. in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;
First, diagnosis of idiopathic pulmonary fibrosis can be 183: 431–40.
challenging and can vary between clinicians as decisions 8 Selman M, King TE, Pardo A, for the American Thoracic Society
Study Group, European Respiratory Society Study Group, and
are solely based on morphological criteria. Second, American College of Chest Physicians Study Group. Idiopathic
prediction of disease behaviour for individual patients is pulmonary fibrosis: prevailing and evolving hypotheses about its
not possible because of considerable interpatient pathogenesis and implications for therapy. Ann Intern Med 2001;
134: 136–51.
heterogeneity. Third, quantification and management of
www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8 9
Seminar
9 Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ, for the Idiopathic 33 Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal
Pulmonary Fibrosis Clinical Research Network Study Group. populations contribute to pulmonary fibrosis without evidence for
Prednisone, azathioprine, and N-acetylcysteine for pulmonary epithelial to mesenchymal transition. Proc Natl Acad Sci USA 2011;
fibrosis. N Engl J Med 2012; 366: 1968–77. 108: E1475–83.
10 Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. 34 Naikawadi RP, Disayabutr S, Mallavia B, et al. Telomere dysfunction
Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. in alveolar epithelial cells causes lung remodeling and fibrosis.
Am J Respir Crit Care Med 1997; 155: 242–48. JCI Insight 2016; 1: e86704.
11 Hubbard R. Occupational dust exposure and the aetiology of 35 Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor
cryptogenic fibrosing alveolitis. Eur Respir J Suppl 2001; for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 2008;
18: 119s–21s. 105: 13051–56.
12 Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an 36 Kropski JA, Pritchett JM, Zoz DF, et al. Extensive phenotyping of
environmental disease? Proc Am Thorac Soc 2006; 3: 293–98. individuals at risk for familial interstitial pneumonia reveals clues
13 Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in to the pathogenesis of interstitial lung disease.
families with idiopathic pulmonary fibrosis. N Engl J Med 2007; Am J Respir Crit Care Med 2015; 191: 417–26.
356: 1317–26. 37 Liang J, Zhang Y, Xie T, et al. Hyaluronan and TLR4 promote
14 Tsakiri KD, Cronkhite JT, Kuan PJ, et al. Adult-onset pulmonary fibrosis surfactant-protein-C- positive alveolar progenitor cell renewal and
caused by mutations in telomerase. Proc Natl Acad Sci USA 2007; prevent severe pulmonary fibrosis in mice. Nat Med 2016;
104: 7552–57. 22: 1285–93.
15 Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant 38 Königshoff M, Kramer M, Balsara N, et al. WNT1-inducible
protein A2 are associated with pulmonary fibrosis and lung cancer. signaling protein–1 mediates pulmonary fibrosis in mice and is
Am J Hum Genet 2009; 84: 52–59. upregulated in humans with idiopathic pulmonary fibrosis.
16 Nogee LM, Dunbar AE, Wert SE, Askin F, Hamvas A, J Clin Invest 2009; 119: 772–87.
Whitsett JA. A mutation in the surfactant protein C gene 39 Stewart GA, Hoyne GF, Ahmad SA, et al. Expression of the
associated with familial interstitial lung disease. N Engl J Med developmental Sonic hedgehog (Shh) signalling pathway is
2001; 344: 573–79. up-regulated in chronic lung fibrosis and the Shh receptor
17 Stuart BD, Choi J, Zaidi S, et al. Exome sequencing links mutations patched 1 is present in circulating T lymphocytes. J Pathol 2003;
in PARN and RTEL1 with familial pulmonary fibrosis and telomere 199: 488–95.
shortening. Nat Genet 2015; 47: 512–17. 40 Horowitz JC, Thannickal VJ. Epithelial-mesenchymal interactions
18 Cogan JD, Kropski JA, Zhao M, et al. Rare variants in RTEL1 are in pulmonary fibrosis. Semin Respir Crit Care Med 2006;
associated with familial interstitial pneumonia. 27: 600–12.
Am J Respir Crit Care Med 2015; 191: 646–55. 41 Hung C, Linn G, Chow Y-H, et al. Role of lung pericytes and
19 Kropski JA, Lawson WE, Young LR, Blackwell TS. Genetic studies resident fibroblasts in the pathogenesis of pulmonary fibrosis.
provide clues on the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2013; 188: 820–30.
Dis Model Mech 2012; 6: 9–17. 42 Liu F, Mih JD, Shea BS, et al. Feedback amplification of fibrosis
20 Fingerlin TE, Murphy E, Zhang W, et al. Genome-wide association through matrix stiffening and COX-2 suppression. J Cell Biol 2010;
study identifies multiple susceptibility loci for pulmonary fibrosis. 190: 693–706.
Nat Genet 2013; 45: 613–20. 43 Booth AJ, Hadley R, Cornett AM, et al. Acellular normal and fibrotic
21 Noth I, Zhang Y, Ma SF, et al. Genetic variants associated with human lung matrices as a culture system for in vitro investigation.
idiopathic pulmonary fibrosis susceptibility and mortality: Am J Respir Crit Care Med 2012; 186: 866–76.
a genome-wide association study. Lancet Respir Med 2013; 1: 309–17. 44 Parker MW, Rossi D, Peterson M, et al. Fibrotic extracellular matrix
22 Evans CM, Fingerlin TE, Schwarz MI, et al. Idiopathic pulmonary activates a profibrotic positive feedback loop. J Clin Invest 2014;
fibrosis: a genetic disease that involves mucociliary dysfunction of 124: 1622–35.
the peripheral airways. Physiol Rev 2016; 96: 1567–91. 45 Tschumperlin DJ. Matrix, mesenchyme, and mechanotransduction.
23 Seibold MA, Wise AL, Speer MC, et al. A common MUC5B Ann Am Thorac Soc 2015; 12: S24–29.
promoter polymorphism and pulmonary fibrosis. N Engl J Med 46 Chen H, Qu J, Huang X, et al. Mechanosensing by the α6-integrin
2011; 364: 1503–12. confers an invasive fibroblast phenotype and mediates lung fibrosis.
24 Hunninghake GM, Hatabu H, Okajima Y, et al. MUC5B promoter Nat Commun 2016; 7: 1–12.
polymorphism and interstitial lung abnormalities. N Engl J Med 2013; 47 Huang X, Yang N, Fiore VF, et al. Matrix stiffness-induced
368: 2192–200. myofibroblast differentiation is mediated by intrinsic
25 Peljto AL, Selman M, Kim DS, et al. The MUC5B promoter mechanotransduction. Am J Respir Cell Mol Biol 2012; 47: 340–48.
polymorphism is associated with IPF in a Mexican cohort but is 48 Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction
rare among Asian ancestries. Chest 2015; 147: 460–64. activates latent TGF- 1 from the extracellular matrix. J Cell Biol 2007;
26 Stock CJ, Sato H, Fonseca C, et al. Mucin 5B promoter 179: 1311–23.
polymorphism is associated with idiopathic pulmonary fibrosis but 49 Zhou Y, Huang X, Hecker L, et al. Inhibition of mechanosensitive
not with development of lung fibrosis in systemic sclerosis or signaling in myofibroblasts ameliorates experimental pulmonary
sarcoidosis. Thorax 2013; 68: 436–41. fibrosis. J Clin Invest 2013; 123: 1096–108.
27 Zhang Y, Noth I, Garcia JGN, Kaminski N. A variant in the 50 Chilosi M, Poletti V, Zamò A, et al. Aberrant Wnt/beta-catenin
promoter of MUC5B and idiopathic pulmonary fibrosis. pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 2003;
N Engl J Med 2011; 364: 1576–77. 162: 1495–502.
28 Roy MG, Livraghi-Butrico A, Fletcher AA, et al. Muc5b is required 51 Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation
for airway defence. Nature 2015; 505: 412–16. of basal and parabasal cells in normal human airway epithelium.
29 Molyneaux PL, Cox MJ, Willis-Owen SAG, et al. The role of bacteria Am J Respir Crit Care Med 1998; 157: 2000–06.
in the pathogenesis and progression of idiopathic pulmonary 52 Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells
fibrosis. Am J Respir Crit Care Med 2014; 190: 906–13. are a multipotent progenitor capable of renewing the bronchial
30 Nakano Y, Yang IV, Walts A, et al. MUC5B promoter variant epithelium. Am J Pathol 2004; 164: 577–88.
rs35705950 affects MUC5B expression in the distal airways in 53 Chilosi M, Poletti V, Murer B, et al. Abnormal re-epithelialization
idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2016; and lung remodeling in idiopathic pulmonary fibrosis: the role
193: 464–66. of ΔN-p63. Lab Invest 2002; 82: 1335–45.
31 Peljto AL, Zhang Y, Fingerlin TE, et al. Association between the 54 Korfei M, Skwarna S, Henneke I, et al. Aberrant expression and
MUC5B promoter polymorphism and survival in patients with activity of histone deacetylases in sporadic idiopathic pulmonary
idiopathic pulmonary fibrosis. JAMA 2013; 309: 2232–39. fibrosis. Thorax 2015; 70: 1022–32.
32 Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and 55 Yang IV, Coldren CD, Leach SM, et al. Expression of
stem cells in lung development, renewal and cancer. Nature 2014; cilium-associated genes defines novel molecular subtypes of
507: 190–94. idiopathic pulmonary fibrosis. Thorax 2013; 68: 114–21.
10 www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8
Seminar
56 King T, Tooze J, Schwarz M, Brown K, Cherniack R. 79 Lancaster LH, Mason WR, Parnell JA, et al. Obstructive sleep apnea
Predicting survival in idiopathic pulmonary fibrosis: scoring system is common in idiopathic pulmonary fibrosis. Chest 2009; 136: 772–78.
and survival model. Am J Respir Crit Care Med 2001; 164: 1171–81. 80 Cottin V, Le Pavec J, Prevot G, et al. Pulmonary hypertension in
57 American Thoracic Society. Idiopathic pulmonary fibrosis: patients with combined pulmonary fibrosis and emphysema
diagnosis and treatment. International consensus statement. syndrome. Eur Respir J 2010; 35: 105–11.
American Thoracic Society (ATS), and the European Respiratory 81 Raghu G, Behr J, Brown KK, et al. Treatment of idiopathic
Society (ERS). Am J Respir Crit Care Med 2000; 161: 646–64. pulmonary fibrosis with ambrisentan: a parallel, randomized trial.
58 American Thoracic Society, European Respiratory Society. Ann Intern Med 2013; 158: 641–49.
American Thoracic Society/European Respiratory Society international 82 Malouf MA, Hopkins P, Snell G, Glanville A. An investigator-driven
multidisciplinary consensus classification of the idiopathic interstitial study of everolimus in surgical lung biopsy confirmed idiopathic
pneumonias. Am J Respir Crit Care Med 2002; 165: 277–304. pulmonary fibrosis. Respirology 2011; 16: 776–83.
59 Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, 83 Noth I, Anstrom KJ, Calvert SB, et al. A placebo-controlled
Remy J. Fleischner Society: glossary of terms for thoracic imaging. randomized trial of warfarin in idiopathic pulmonary fibrosis.
Radiology 2008; 246: 697–722. Am J Respir Crit Care Med 2012; 186: 88–95.
60 Travis WD, Costabel U, Hansell DM, et al. An official American 84 King TE Jr, Brown KK, Raghu G, et al. BUILD-3: a randomized,
Thoracic Society/European Respiratory Society statement: update of controlled trial of bosentan in idiopathic pulmonary fibrosis.
the international multidisciplinary classification of the idiopathic Am J Respir Crit Care Med 2011; 184: 92–99.
interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–48. 85 Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E,
61 Hunninghake GW, Lynch DA, Galvin JR, et al. Radiologic findings Schroeder DR. Imatinib treatment for idiopathic pulmonary
are strongly associated with a pathologic diagnosis of usual fibrosis. Am J Respir Crit Care Med 2010; 181: 604–10.
interstitial pneumonia. Chest 2003; 124: 1215–23. 86 Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J,
62 Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. for the MUSIC study group. Macitentan for the treatment of
In-hospital mortality following surgical lung biopsy for interstitial idiopathic pulmonary fibrosis: the randomised controlled MUSIC
lung disease in the USA: 2000–2011. Am J Respir Crit Care Med trial. Eur Respir J 2013; 42: 1622–32.
2016; 193: 1161–67. 87 Martinez FJ, de Andrade JA, Anstrom KJ, King TE Jr, Raghu G,
63 Fell CD, Martinez FJ, Liu LX, et al. Clinical predictors of a diagnosis for the Idiopathic Pulmonary Fibrosis Clinical Research Network
of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2010; Study Group. Randomized trial of acetylcysteine in idiopathic
181: 832–37. pulmonary fibrosis. N Engl J Med 2014; 370: 2093–101.
64 Brownell R, Moua T, Henry TS, et al. The use of pretest probability 88 Zisman DA, Schwarz M, Schwarz M, Anstrom KJ, Collard HR,
increases the value of high-resolution CT in diagnosing usual Flaherty KR, Hunninghake GW, for the Idiopathic Pulmonary
interstitial pneumonia. Thorax 2017; published online Jan 12. Fibrosis Clinical Research Network, Study Group. A controlled trial
DOI:10.1136/thoraxjnl-2016-209671. of sildenafil in advanced idiopathic pulmonary fibrosis.
65 Jones MG, Fabre A, Schneider P, et al. Three-dimensional N Engl J Med 2010; 363: 620–28
characterization of fibroblast foci in idiopathic pulmonary fibrosis. 89 Richeldi L, Bois du RM, Raghu G, et al. Efficacy and safety of
JCI Insight 2016; 1: e86375. nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;
66 Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, 370: 2071–82.
Brown KK. Fibroblast foci are not discrete sites of lung injury or 90 King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial
repair: the fibroblast reticulum. Am J Respir Crit Care Med 2006; of pirfenidone in patients with idiopathic pulmonary fibrosis.
174: 654–58. N Engl J Med 2014; 370: 2083–92.
67 Flaherty KR, King TE Jr, Raghu G, et al. Idiopathic interstitial 91 Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients
pneumonia: what is the effect of a multidisciplinary approach to with idiopathic pulmonary fibrosis (CAPACITY): two randomised
diagnosis? Am J Respir Crit Care Med 2004; 170: 904–10. trials. Lancet 2011; 377: 1760–69.
68 Ryerson CJ, Urbania TH, Richeldi L, et al. Prevalence and prognosis of 92 Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase
unclassifiable interstitial lung disease. Eur Respir J 2013; 42: 750–57. inhibitor with sustained receptor blockade and good antitumor
69 Bois du RM, Weycker D, Albera C, et al. Ascertainment of efficacy. Cancer Res 2008; 68: 4774–82.
individual risk of mortality for patients with idiopathic pulmonary 93 Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF,
fibrosis. Am J Respir Crit Care Med 2011; 184: 459–66. VEGF and FGF signalling attenuates fibrosis. Eur Respir J 2007;
70 Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index 29: 976–85.
and staging system for idiopathic pulmonary fibrosis. 94 Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine
Ann Intern Med 2012; 156: 684–91. kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med
71 Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: 2011; 365: 1079–87.
a composite physiologic index derived from disease extent observed by 95 Costabel U, Inoue Y, Richeldi L, et al. Efficacy of nintedanib in
computed tomography. Am J Respir Crit Care Med 2003; 167: 962–69. idiopathic pulmonary fibrosis across prespecified subgroups in
72 Kropski JA, Young LR, Cogan JD, et al. Genetic evaluation and INPULSIS. Am J Respir Crit Care Med 2016; 193: 178–85.
testing of patients and families with idiopathic pulmonary fibrosis. 96 Lee BS, Margolin SB, Nowak RA. Pirfenidone: a novel
Am J Respir Crit Care Med 2016; published online Oct 27. pharmacological agent that inhibits leiomyoma cell proliferation
DOI:10.1164/rccm.201609-1820PP. and collagen production. J Clin Endocrinol Metab 1998; 83: 219–23.
73 Talbert JL, Schwartz DA, Steele MP. Familial interstitial pneumonia 97 Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K.
(FIP). Clin Pulm Med 2014; 21: 120–27. Antifibrotic activities of pirfenidone in animal models.
74 Holohan B, Wright WE, Shay JW. Telomeropathies: an emerging Eur Respir Rev 2011; 20: 85–97.
spectrum disorder. J Cell Biol 2014; 205: 289–99. 98 Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic
75 Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. pulmonary fibrosis. Eur Respir J 2010; 35: 821–29.
Delayed access and survival in idiopathic pulmonary fibrosis. 99 Noble PW, Bradford WZ, Costabel U, et al. Pirfenidone for
Am J Respir Crit Care Med 2011; 184: 842–47. idiopathic pulmonary fibrosis: analysis of pooled data from
76 Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ three multinational phase 3 trials. Eur Respir J 2016; 47: 27–30.
ALAT clinical practice guideline: treatment of idiopathic pulmonary 100 European Medicines Agency. Pirfenidone. Annex I: summary of
fibrosis. An update of the 2011 clinical practice guideline. product characteristics. 2015. http://www.ema.europa.eu/docs/en_
Am J Respir Crit Care Med 2015; 192: e3–19. GB/document_library/EPAR_-_Product_Information/
77 Raghu G, Behr J, Stowasser S. Comorbidities in idiopathic human/002154/WC500103049.pdf (accessed Oct 1, 2016).
pulmonary fibrosis patients: a systematic literature review. 101 European Medicines Agency. Nintedanib. Annex I: summary of
Eur Respir J 2015; 46: 1113–30. product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/
78 Cottin V. The impact of emphysema in pulmonary fibrosis. document_library/EPAR_-_Product_Information/human/002569/
Eur Respir Rev 2013; 22: 153–57. WC500179970.pdf (accessed Oct 1, 2016).
www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8 11
Seminar
102 Schmidinger M. Understanding and managing toxicities of vascular 122 National Institute for Health and Care Excellence. Technology
endothelial growth factor (VEGF) inhibitors. EJC Suppl 2013; appraisal guidance TA282. Pirfenidone for treating idioapthic
11: 172–91. pulmonary fibrosis. 2013. https://www.nice.org.uk/guidance/ta282
103 Kistler KD, Nalysnyk L, Rotella P, Esser D. Lung transplantation in (accessed Oct 1, 2016).
idiopathic pulmonary fibrosis: a systematic review of the literature. 123 Oldham JM, Ma SF, Martinez FJ, et al. TOLLIP, MUC5B and the
BMC Pulm Med 2014; 14: 788. response to N-acetylcysteine among Individuals with idiopathic
104 Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of pulmonary fibrosis. Am J Respir Crit Care Med 2015; 192: 1475–82.
idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007; 124 Tobin RW, Pope CE, Pellegrini CA, Emond MJ, Sillery J, Raghu G.
176: 636–43. Increased prevalence of gastroesophageal reflux in patients with
105 Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;
idiopathic pulmonary fibrosis. An international working group 158: 1804–08.
report. Am J Respir Crit Care Med 2016; 194: 265–75. 125 Lee JS, Collard HR, Raghu G, et al. Does chronic microaspiration
106 Parambil J, Myers J, Ryu JH. Histopathologic features and outcome cause idiopathic pulmonary fibrosis? Am J Med 2010; 123: 304–11.
of patients with acute exacerbation of idiopathic pulmonary fibrosis 126 Lee JS, Ryu JH, Elicker BM, et al. Gastroesophageal reflux therapy
undergoing surgical lung biopsy. Chest 2015; 128: 3310–15. is associated with longer survival in patients with idiopathic
107 Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of pulmonary fibrosis. Am J Respir Crit Care Med 2011; 184: 1390–94.
idiopathic pulmonary fibrosis: incidence, risk factors and outcome. 127 Lee JS, Collard HR, Anstrom KJ, et al. Anti-acid treatment and
Eur Respir J 2011; 37: 356–63. disease progression in idiopathic pulmonary fibrosis: an analysis of
108 Bajwah S, Higginson IJ, Ross JR, et al. Specialist palliative care is data from three randomised controlled trials. Lancet Respir Med
more than drugs: a retrospective study of ILD patients. Lung 2012; 2013; 1: 369–76.
190: 215–20. 128 Kreuter M, Wuyts W, Renzoni E, et al. Antacid therapy and disease
109 Bajwah S, Ross JR, Peacock JL, et al. Interventions to improve outcomes in idiopathic pulmonary fibrosis: a pooled analysis.
symptoms and quality of life of patients with fibrotic interstitial Lancet Respir Med 2016; 4: 381–89.
lung disease: a systematic review of the literature. Thorax 2013; 129 Raghu G, Morrow E, Collins BF, et al. Laparoscopic anti-reflux
68: 867–79. surgery for idiopathic pulmonary fibrosis at a single centre.
110 Hope-Gill BDM, Hilldrup S, Davies C, Newton RP, Harrison NK. Eur Respir J 2016; 48: 826–32.
A study of the cough reflex in idiopathic pulmonary fibrosis. 130 Han MK, Zhou Y, Murray S, et al. Lung microbiome and disease
Am J Respir Crit Care Med 2003; 168: 995–1002. progression in idiopathic pulmonary fibrosis: an analysis of the
111 Ryerson CJ, Cayou C, Topp F, et al. Pulmonary rehabilitation COMET study. Lancet Respir Med 2014; 2: 548–6.
improves long-term outcomes in interstitial lung disease: 131 Shulgina LL, Cahn APA, Chilvers ERE, et al. Treating idiopathic
a prospective cohort study. Respir Med 2014; 108: 203–10. pulmonary fibrosis with the addition of co-trimoxazole:
112 Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for a randomised controlled trial. Thorax 2013; 68: 155–62.
interstitial lung disease. Cochrane Database Syst Rev 2014; 132 Varney VA, Parnell HM, Salisbury DT, Ratnatheepan S, Tayar RB.
10: CD006322. A double blind randomised placebo controlled pilot study of oral
113 Swigris JJ, Fairclough DL, Morrison M, et al. Benefits of pulmonary co-trimoxazole in advanced fibrotic lung disease.
rehabilitation in idiopathic pulmonary fibrosis. Respir Care 2011; Pulm Pharmacol Ther 2008; 21: 178–87.
56: 783–89. 133 Jenkins RG, Simpson JK, Saini G, et al. Longitudinal change in
114 Raghu G, Wells AU, Nicholson AG, et al. Effect of nintedanib in collagen degradation biomarkers in idiopathic pulmonary fibrosis:
subgroups of idiopathic pulmonary fibrosis by diagnostic criteria. an analysis from the prospective, multicentre PROFILE study.
Am J Respir Crit Care Med 2017; 195: 78–85. Lancet Respir Med 2015; 3: 462–72.
115 Walsh SLF, Wells AU, Desai SR, et al. Multicentre evaluation of 134 Rosas IO, Richards TJ, Konishi K, et al. MMP1 and MMP7 as
multidisciplinary team meeting agreement on diagnosis in diffuse potential peripheral blood biomarkers in idiopathic pulmonary
parenchymal lung disease: a case-cohort study. Lancet Respir Med 2016; fibrosis. PLoS Med 2008; 5: e93.
4: 557–65. 135 O’Dwyer DN, Armstrong ME, Trujillo G, et al. The toll-like
116 Walsh SLF, Calandriello L, Sverzellati N, Wells AU, Hansell DM, receptor 3 L412F polymorphism and disease progression in
for the UIP Observer Consort Study Group. Interobserver idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2013;
agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern 188: 1442–50.
on CT. Thorax 2016; 71: 45–51. 136 Herazo-Maya JD, Noth I, Duncan SR, et al. Peripheral blood
117 Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic lung mononuclear cell gene expression profiles predict poor outcome in
cryobiopsy increases diagnostic confidence in the multidisciplinary idiopathic pulmonary fibrosis. Sci Transl Med 2013; 5: 205ra136.
diagnosis of idiopathic pulmonary fibrosis. 137 Prasse A, Probst C, Bargagli E, et al. Serum CC-chemokine ligand
Am J Respir Crit Care Med 2016; 193: 745–52. 18 concentration predicts outcome in idiopathic pulmonary fibrosis.
118 Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Am J Respir Crit Care Med 2009; 179: 717–23.
Diagnostic yield and complications of transbronchial lung 138 Behr J, Bendstrup E, Crestani B, et al. Safety and tolerability of
cryobiopsy for interstitial lung disease: a systematic review and acetylcysteine and pirfenidone combination therapy in idiopathic
meta-analysis. Ann Am Thorac Soc 2016; 13: 1828–38. pulmonary fibrosis: a randomised, double-blind, placebo-controlled,
119 Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic phase 2 trial. Lancet Respir Med 2016; 4: 445–53.
pulmonary fibrosis—FDA review of pirfenidone and nintedanib. 139 Ogura A, Taniguchi H, Azuma A, et al. Safety and pharmacokinetics
N Engl J Med 2015; 372: 1189–91. of nintedanib and pirfenidone in idiopathic pulmonary fibrosis.
120 Albera C, Fagan EA, Glassberg MK, et al. Efficacy of pirfenidone in Eur Respir J 2015; 45: 1382–92.
patients with idiopathic pulmonary fibrosis with more preserved 140 Rockey DC, Bell PD, Hill JA. Fibrosis—a common pathway to organ
lung function. Eur Respir J 2016; 48: 843–51. injury and failure. N Engl J Med 2015; 372: 1138–49.
121 Nathan SD, Albera C, Bradford WZ, et al. Effect of continued
treatment with pirfenidone following clinically meaningful declines
in forced vital capacity: analysis of data from three phase 3 trials in
patients with idiopathic pulmonary fibrosis. Thorax 2016; 71: 429–35.
12 www.thelancet.com Published online March 29, 2017 http://dx.doi.org/10.1016/S0140-6736(17)30866-8
You might also like
- Stem Cell-Based Therapy for Lung DiseaseFrom EverandStem Cell-Based Therapy for Lung DiseaseJanette K. BurgessNo ratings yet
- Nathan 2020Document12 pagesNathan 2020gustavo adolfo rodriguez alzateNo ratings yet
- Idiopathic Pulmonary Fibrosis: Diagnosis, Epidemiology and Natural HistoryDocument11 pagesIdiopathic Pulmonary Fibrosis: Diagnosis, Epidemiology and Natural HistorySuwandi AlghozyNo ratings yet
- Fgene 10 01327Document10 pagesFgene 10 01327Serque777No ratings yet
- Asthma Lancet 23febDocument18 pagesAsthma Lancet 23febMr. LNo ratings yet
- 1 s2.0 S0140673622010522 MainDocument18 pages1 s2.0 S0140673622010522 MainsilviaNo ratings yet
- Idiopathic Pulmonary FibrosisDocument13 pagesIdiopathic Pulmonary FibrosisRaul David Maldonado LearyNo ratings yet
- Review: Paolo Spagnolo, Giulio Rossi, Rocco Trisolini, Nicola Sverzellati, Robert P Baughman, Athol U WellsDocument14 pagesReview: Paolo Spagnolo, Giulio Rossi, Rocco Trisolini, Nicola Sverzellati, Robert P Baughman, Athol U Wellsandra mNo ratings yet
- 1 s2.0 S0140673622010522 MainDocument18 pages1 s2.0 S0140673622010522 MainMirella Rugel SocolaNo ratings yet
- Idiopathic Pulmonary Fibrosis: Optimizing The Diagnosis and Multi-Disciplinary Decision MakingDocument4 pagesIdiopathic Pulmonary Fibrosis: Optimizing The Diagnosis and Multi-Disciplinary Decision MakingAnna LiachenkoNo ratings yet
- Fpi Nejm Clincal Practice 2018Document13 pagesFpi Nejm Clincal Practice 2018cjonderedsonNo ratings yet
- Latest IipDocument7 pagesLatest IipPULMONOLOGI 10 USKNo ratings yet
- ALVIRA 2017_Can We Understand the Pathobiology of Bronchopulmonary DysplasiaDocument11 pagesALVIRA 2017_Can We Understand the Pathobiology of Bronchopulmonary DysplasiaRafael JustinoNo ratings yet
- AsthmaDocument18 pagesAsthmaMihaela BerindeieNo ratings yet
- Lung Involvement in Childhood Measles: Severe Immune Dysfunction Revealed by Quantitative ImmunohistochemistryDocument10 pagesLung Involvement in Childhood Measles: Severe Immune Dysfunction Revealed by Quantitative Immunohistochemistryapi-286128974No ratings yet
- Role of Multi-Detector Computed Tomography in Evaluation of Lung AnomaliesDocument16 pagesRole of Multi-Detector Computed Tomography in Evaluation of Lung AnomaliesIJAR JOURNALNo ratings yet
- Bio Marc AdoresDocument21 pagesBio Marc AdoresJanet Victoria Maturano MartínezNo ratings yet
- Barratt 2018Document21 pagesBarratt 2018Examenes LaboratorioNo ratings yet
- EditorialDocument2 pagesEditorialsebastoto778No ratings yet
- Fungal EndocarditisDocument4 pagesFungal EndocarditisJorgeNo ratings yet
- Pleural Infection - Past, Present, and Future DirectionsDocument15 pagesPleural Infection - Past, Present, and Future DirectionsCamilo VidalNo ratings yet
- Diagnosis and Management of Adrenal InsufficiencyDocument11 pagesDiagnosis and Management of Adrenal Insufficiencyinterna MANADONo ratings yet
- FPI Progn TratDocument13 pagesFPI Progn TratTurcu AndreeaNo ratings yet
- Primer: BronchiectasisDocument18 pagesPrimer: BronchiectasisAnanta Bryan Tohari WijayaNo ratings yet
- Cell-Based Therapy To Reduce Mortality From COVID-19Document16 pagesCell-Based Therapy To Reduce Mortality From COVID-19Chelsea Shannen Marie CarreonNo ratings yet
- N Engl J Med 2022 386 1058Document12 pagesN Engl J Med 2022 386 1058EvelynNo ratings yet
- Cryptogenic Organizing Pneumonia: Review ArticleDocument12 pagesCryptogenic Organizing Pneumonia: Review ArticleMatías Jesús Flamm ZamoranoNo ratings yet
- 2019 Polución de Aire y Enfermedades No Comunicables Parte 2Document10 pages2019 Polución de Aire y Enfermedades No Comunicables Parte 2Marcelo Ortiz ValenzuelaNo ratings yet
- Reviewing Atrial Fibrillation Pathophysiology FromDocument12 pagesReviewing Atrial Fibrillation Pathophysiology From23Riya Khot VcetNo ratings yet
- Cryptogenic Organizing PneumoniaDocument12 pagesCryptogenic Organizing Pneumoniajerodussart.22No ratings yet
- Ioi80197 463 473Document11 pagesIoi80197 463 473Phạm LinhNo ratings yet
- 410-1216-1-PBDocument2 pages410-1216-1-PBNousradine MournoNo ratings yet
- Clin Infect Dis. 2004 Boggild 1123 8Document6 pagesClin Infect Dis. 2004 Boggild 1123 8Arya PratamaNo ratings yet
- Sjögren's Syndrome: From Pathogenesis To Novel Therapeutic TargetsDocument5 pagesSjögren's Syndrome: From Pathogenesis To Novel Therapeutic TargetsOcha24 TupamahuNo ratings yet
- Neumococo en NacDocument15 pagesNeumococo en NacMNo ratings yet
- Chronic Ethanol Consumption Compromises Neutrophil Function in Acute Pulmonary Aspergillus Fumigatus InfectionDocument23 pagesChronic Ethanol Consumption Compromises Neutrophil Function in Acute Pulmonary Aspergillus Fumigatus InfectionTassia PontesNo ratings yet
- Pone 0234306 PDFDocument18 pagesPone 0234306 PDFyenny handayani sihiteNo ratings yet
- 34 Iajps34102020Document7 pages34 Iajps34102020iajpsNo ratings yet
- Cryptogenic Organizing PneumoniaDocument12 pagesCryptogenic Organizing Pneumoniavalentina peñaNo ratings yet
- Multiple Sclerosis Lancet 2018Document15 pagesMultiple Sclerosis Lancet 2018Sarah Miryam CoffanoNo ratings yet
- Di¡erential Adaptation of Microbial Pathogens To Airways of Patients With Cystic Brosis and Chronic Obstructive Pulmonary DiseaseDocument24 pagesDi¡erential Adaptation of Microbial Pathogens To Airways of Patients With Cystic Brosis and Chronic Obstructive Pulmonary DiseaseAbd Halim Gazali HNo ratings yet
- DR Syed Aqeel Gilani Department of Pulmonology Ayub Teaching Hospital AbbottabadDocument49 pagesDR Syed Aqeel Gilani Department of Pulmonology Ayub Teaching Hospital AbbottabadAnka EremiaNo ratings yet
- ORENSTEIN 2002_Cystic fibrosis- A 2002 updateDocument9 pagesORENSTEIN 2002_Cystic fibrosis- A 2002 updateRafael JustinoNo ratings yet
- Biomedicines 11 01636 v2Document14 pagesBiomedicines 11 01636 v2Diana CalarasNo ratings yet
- Organising PneumoniaDocument12 pagesOrganising PneumoniaA. RaufNo ratings yet
- Asthma Without Borders: EditorialDocument3 pagesAsthma Without Borders: EditorialHesbon MomanyiNo ratings yet
- Microparticulas ArdsDocument18 pagesMicroparticulas ArdsARELHI ROSARIO GARCIA MONROYNo ratings yet
- Allergy - 2019 - Ivanova - What Did We Learn From Multiple Omics Studies in AsthmaDocument17 pagesAllergy - 2019 - Ivanova - What Did We Learn From Multiple Omics Studies in Asthmanhc_king_proNo ratings yet
- Interstitial Pneumonia Seminar PaperDocument9 pagesInterstitial Pneumonia Seminar PaperLamy SNo ratings yet
- Chronic Interstitial Lung Diseases in Children: Diagnosis ApproachesDocument30 pagesChronic Interstitial Lung Diseases in Children: Diagnosis Approachesgustavo adolfo rodriguez alzateNo ratings yet
- ASIP Centennial Review: Cerebral MalariaDocument9 pagesASIP Centennial Review: Cerebral MalariaAndi Karwana CiptaNo ratings yet
- Piy 008Document4 pagesPiy 008Alejandro GutiérrezNo ratings yet
- Update in Diagnosis and Management of Interstitial Lung DiseaseDocument8 pagesUpdate in Diagnosis and Management of Interstitial Lung DiseaseTimothy23 SiregarNo ratings yet
- Adult Interstitial Lung Diseases and Their Epidemiology 2019Document46 pagesAdult Interstitial Lung Diseases and Their Epidemiology 2019Mary CogolloNo ratings yet
- ENG JCOPDF-2019-0128-StringerDocument11 pagesENG JCOPDF-2019-0128-Stringerulfiandi rizkiNo ratings yet
- Difficulties in Diagnosis of Psittacosis or Ornithosis: A Case ReportDocument4 pagesDifficulties in Diagnosis of Psittacosis or Ornithosis: A Case ReportYusrinabillaNo ratings yet
- Factors Predicting Mortality in Leptospirosis PatientsDocument8 pagesFactors Predicting Mortality in Leptospirosis PatientsSilvia RNo ratings yet
- Leeuw en 2014Document7 pagesLeeuw en 2014Elisabeth Agnes SidabutarNo ratings yet
- European Consensus For The Management of Patients With Differentiated Thyroid Carcinoma of The Follicular EpitheliumDocument17 pagesEuropean Consensus For The Management of Patients With Differentiated Thyroid Carcinoma of The Follicular Epitheliumelibet87No ratings yet
- Jem 20130084Document12 pagesJem 20130084Vivien WuNo ratings yet
- S S, Baba MRDocument7 pagesS S, Baba MRPrasasti 19No ratings yet
- Stroke AFRDocument9 pagesStroke AFRPrasasti 19No ratings yet
- 8380 40823 3 PBDocument10 pages8380 40823 3 PBbima ardyantoNo ratings yet
- Measurement of The Angle of Plantar Flexion An Objective Way of Assessing Muscle Relaxation in Children With Spastic Cerebral PalsyDocument5 pagesMeasurement of The Angle of Plantar Flexion An Objective Way of Assessing Muscle Relaxation in Children With Spastic Cerebral PalsyPrasasti 19No ratings yet
- Modified Ashworth Scale Instructions PDFDocument3 pagesModified Ashworth Scale Instructions PDFPrasasti 19No ratings yet
- The Role of Pulmonary Rehabilitation in Patients With Idiopathic Pulmonary FibrosisDocument25 pagesThe Role of Pulmonary Rehabilitation in Patients With Idiopathic Pulmonary FibrosisPrasasti 19No ratings yet
- Mutlu2008 PDFDocument8 pagesMutlu2008 PDFmilhimNo ratings yet
- Artificial Neural Network Learns Clinical Assessment of Spasticity in Modified Ashworth ScaleDocument9 pagesArtificial Neural Network Learns Clinical Assessment of Spasticity in Modified Ashworth ScalePrasasti 19No ratings yet
- Efficacy of Adding Activity of Daily Living Simulation Training To Traditional Pulmonary Rehabilitation On Dyspnea and Health-Related Quality-Of-LifeDocument11 pagesEfficacy of Adding Activity of Daily Living Simulation Training To Traditional Pulmonary Rehabilitation On Dyspnea and Health-Related Quality-Of-LifePrasasti 19No ratings yet
- Modified Ashworth Scale MAS Model Based On ClinicaDocument8 pagesModified Ashworth Scale MAS Model Based On ClinicaPrasasti 19No ratings yet
- Pulmonary Rehabilitation in Interstitial Lung Diseases: ReviewDocument7 pagesPulmonary Rehabilitation in Interstitial Lung Diseases: ReviewPrasasti 19No ratings yet
- Motivation Letter SafiraDocument3 pagesMotivation Letter SafiraPrasasti 19No ratings yet
- Combined Pulmonary Fibrosis and Emphysema: Effect of Pulmonary Rehabilitation in Comparison With Chronic Obstructive Pulmonary DiseaseDocument10 pagesCombined Pulmonary Fibrosis and Emphysema: Effect of Pulmonary Rehabilitation in Comparison With Chronic Obstructive Pulmonary DiseasePrasasti 19No ratings yet
- Respiratory Interventions For Breathlessness in Adults With Advanced Diseases (Protocol)Document15 pagesRespiratory Interventions For Breathlessness in Adults With Advanced Diseases (Protocol)Prasasti 19No ratings yet
- Aerobic and Breathing Exercises Improve Dyspnea, Exercise Capacity and Quality of Life in Idiopathic Pulmonary Fibrosis Patients: Systematic Review and Meta-AnalysisDocument16 pagesAerobic and Breathing Exercises Improve Dyspnea, Exercise Capacity and Quality of Life in Idiopathic Pulmonary Fibrosis Patients: Systematic Review and Meta-AnalysisPrasasti 19No ratings yet
- Stroke AFRDocument9 pagesStroke AFRPrasasti 19No ratings yet
- S S, Baba MRDocument7 pagesS S, Baba MRPrasasti 19No ratings yet
- (Mungkin Jadi) - EssayDocument2 pages(Mungkin Jadi) - EssayPrasasti 19No ratings yet
- Artikel 20engmed 20haura 20tamaDocument3 pagesArtikel 20engmed 20haura 20tamaPrasasti 19No ratings yet
- PTJ 1905Document14 pagesPTJ 1905Prasasti 19No ratings yet
- Cerebral PalsyDocument2 pagesCerebral PalsyPrasasti 19No ratings yet
- Free Local Food PPT Templates: Insert The Subtitle of Your PresentationDocument48 pagesFree Local Food PPT Templates: Insert The Subtitle of Your PresentationJoan RamirezNo ratings yet
- Medical Health Care PowerPoint TemplatesDocument48 pagesMedical Health Care PowerPoint TemplatesAl-UmamNo ratings yet
- Prasasti - TOEIC Listening and Reading Test ResultsDocument3 pagesPrasasti - TOEIC Listening and Reading Test ResultsPrasasti 19No ratings yet
- Naskah Publikasi PDFDocument15 pagesNaskah Publikasi PDFPrasasti 19No ratings yet
- Tidys Fracture PDFDocument26 pagesTidys Fracture PDFPrasasti 19No ratings yet
- Cryptogenic Organizing PneumoniaDocument12 pagesCryptogenic Organizing Pneumoniavalentina peñaNo ratings yet
- Interstitial Lung DiseaseDocument77 pagesInterstitial Lung DiseaseMahaveer S ShekhawatNo ratings yet
- HRCT Protocols & Artifacts: Leena. R. David M.SC - MITDocument24 pagesHRCT Protocols & Artifacts: Leena. R. David M.SC - MITNadeem ShahNo ratings yet
- Pulmonary Involvement in Antisynthetase Syndrome 2019Document8 pagesPulmonary Involvement in Antisynthetase Syndrome 2019yokotoyNo ratings yet
- 85402-100 ERBE EN ERBECRYO 2 With Flexible Cryoprobes D071400Document12 pages85402-100 ERBE EN ERBECRYO 2 With Flexible Cryoprobes D071400Hossain TanjilaaNo ratings yet
- Arya - Usefulness and Safety of Transbronchial Biopsy With Large Forceps During Flexible BronchosDocument5 pagesArya - Usefulness and Safety of Transbronchial Biopsy With Large Forceps During Flexible BronchosXaralyn XaviereNo ratings yet
- The Essential Guide To The New FRCR Part 2A and Radiology BoardsDocument208 pagesThe Essential Guide To The New FRCR Part 2A and Radiology Boardsbana galaxyNo ratings yet
- Path MCQ 2003-2005 RecallsDocument91 pagesPath MCQ 2003-2005 RecallsLuthie Singh100% (1)
- Cavitary Lesions of Lung - S - 12 June - SSTDocument118 pagesCavitary Lesions of Lung - S - 12 June - SSTManu ChopraNo ratings yet
- Radiology - AFIP - 2006-2007 Lecture NotesDocument1,594 pagesRadiology - AFIP - 2006-2007 Lecture Notesjvmeyer100% (1)
- Mksap PulmonaryDocument77 pagesMksap PulmonaryAna Roman100% (4)
- Diagnosing Pulmonary Edema with Chest Radiography, Lung Ultrasound and CTDocument5 pagesDiagnosing Pulmonary Edema with Chest Radiography, Lung Ultrasound and CTKen FcNo ratings yet
- Chapter 5 - Recognizing Airspace Versus Interstitial Lung DiseaseDocument19 pagesChapter 5 - Recognizing Airspace Versus Interstitial Lung DiseaseRisky IndraNo ratings yet
- PresentasiDocument30 pagesPresentasiagusNo ratings yet
- Amiodarone Tablets for ArrhythmiasDocument10 pagesAmiodarone Tablets for Arrhythmiasddandan_2No ratings yet
- Cyst and Cystlike Lung LesionsDocument57 pagesCyst and Cystlike Lung LesionsAna StankovicNo ratings yet
- Breathing in AmericaDocument282 pagesBreathing in AmericaGakTauMauKasiNamaApaNo ratings yet
- 2013 July Chest Section PDFDocument171 pages2013 July Chest Section PDFsebsbie admasNo ratings yet
- Hamman Rich SyndromeDocument7 pagesHamman Rich SyndromeGerardo Armando EsparzaNo ratings yet
- Pulmonary PathophysiologyDocument146 pagesPulmonary PathophysiologySeeta NanooNo ratings yet
- Marquis Et Al 2023 CT Approach To Lung InjuryDocument19 pagesMarquis Et Al 2023 CT Approach To Lung InjuryMinh đăng ĐỗNo ratings yet
- Plain Film and HRCT Diagnosis of Interstitial Lung Disease: Sujal R. Desai, Helmut Prosch, and Jeffrey R. GalvinDocument9 pagesPlain Film and HRCT Diagnosis of Interstitial Lung Disease: Sujal R. Desai, Helmut Prosch, and Jeffrey R. GalvinVictor ChiabaiNo ratings yet
- Connective Tissue Disease-Associated Interstitial Lung DiseaseDocument35 pagesConnective Tissue Disease-Associated Interstitial Lung DiseaseSilp SatjawattanavimolNo ratings yet
- 5 6287273689696174255Document318 pages5 6287273689696174255rani silahudinNo ratings yet
- Pathogenesis and Pathology of Bovine Pne PDFDocument24 pagesPathogenesis and Pathology of Bovine Pne PDFKaran VetNo ratings yet
- Afectarea Pulmonară Din Diverse Boli Reumatice: Dr. Corina MogosanDocument25 pagesAfectarea Pulmonară Din Diverse Boli Reumatice: Dr. Corina MogosanCioca ElizaNo ratings yet
- deocument_936Full download book Practical Pulmonary Pathology A Diagnostic Approach A Volume In The Pattern Recognition Series Pdf pdfDocument41 pagesdeocument_936Full download book Practical Pulmonary Pathology A Diagnostic Approach A Volume In The Pattern Recognition Series Pdf pdfmark.king621100% (12)
- HY PulmonaryDocument67 pagesHY PulmonaryJeniNo ratings yet
- Ac 76583 Pfdqwertaf 79898 TseDocument548 pagesAc 76583 Pfdqwertaf 79898 TseMerve EmelNo ratings yet
- Icd 10Document24 pagesIcd 10Mary Conway0% (1)