Professional Documents
Culture Documents
Hydrogen Production
Uploaded by
Thanigaivel A0 ratings0% found this document useful (0 votes)
29 views10 pagesCopyright
© © All Rights Reserved
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document0 ratings0% found this document useful (0 votes)
29 views10 pagesHydrogen Production
Uploaded by
Thanigaivel AYou are on page 1of 10
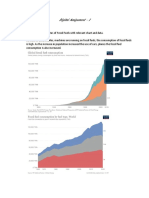
There are four main sources for the
commercial production of hydrogen:
natural gas, oil, coal, and electrolysis;
which account for 48%, 30%, 18%
and 4% of the world’s hydrogen
production respectively.
Thermochemical Processes
Thermochemical processes use heat and
chemical reactions to release hydrogen from
organic materials such as fossil fuels and
biomass.
Some thermal processes use the energy in
various resources, such as natural gas, coal, or
biomass, to release hydrogen from their
molecular structure.
In other processes, heat, in combination with
closed-chemical cycles, produces hydrogen from
feedstocks such as water.
Steam Reforming
For this process high temperature (700–1100 °C) steam
(H2O) reacts with methane (CH4) in an endothermic
reaction to yield syngas.
CH4 + H2O + heat → CO + 3 H2
In a second stage, additional hydrogen is generated
through the lower-temperature, exothermic, water gas
shift reaction, performed at about 360 °C:
CO + H2O → CO2 + H2 + heat
Essentially, the oxygen (O) atom is stripped from the
additional water (steam) to oxidize CO to CO2. This
oxidation also provides energy to maintain the reaction.
Additional heat required to drive the process is generally
supplied by burning some portion of the methane.
Direct Solar Water Splitting Processes
Direct solar water splitting, or photolytic,
processes use light energy to split water into
hydrogen and oxygen.
These processes are currently in the very early
stages of research but offer long-term potential
for sustainable hydrogen production with low
environmental impact.
Biological Processes
Microbes such as bacteria and microalgae can
produce hydrogen through biological reactions,
using sunlight or organic matter.
These technology pathways are at an early
stage of research, but in the long term have
the potential for sustainable, low-carbon
hydrogen production.
Hydrogen:
Hydrogen has the highest energy content per unit of mass of
any chemical fuel
It can substitute for hydrocarbons in a broad range of
application
Its combustion efficiency is higher
It can be used as fuel directly or can be used as a raw material
to produce methanol, ammonia, or hydrocarbons by using
either carbon dioxide or nitrogen from the atmosphere.
Hydrogen is chemically very reactive and hence it is not found
in its free state on the earth.
At standard temperature and pressure,
hydrogen is a colorless
tasteless
odorless gas
Hydrogen gas is highly flammable and will burn in air
in concentrations between 4% and 75% by volume
Hydrogen is not toxic, but in its pure form is a chemical
asphyxiant.
An asphyxiant gas is a nontoxic or minimally toxic gas which reduces or
displaces the normal oxygen concentration in breathing air. Breathing of
oxygen-depleted air can lead to death by asphyxiation (suffocation).
Hydrogen gas leaking into air may spontaneously
ignite
Electrolysis:
A source of direct current voltage is connected to the
electrodes so that an electric current flows through the
electrolyte from the positive electrode (or anode) to the
negative electrode (or cathode).
As a result the water in the electrolyte solution is
decomposed into hydrogen gas (H2) which is released at the
cathode and oxygen gas (O2) is released at the anode.
KOH solution (electrolyte) is required because water is very
poor conductor of electricity.
Ideally, a voltage of 1.23 volts should be sufficient for the
electrolysis of water at normal temperature and pressure.
The rate of hydrogen production is proportional to the
current strength, a high operating current density is
necessary for economic reasons.
Theoretically, 2.8 kW-hr of electrical energy should produce
one cu.m of hydrogen gas.
Hydrogen storage methods:
Compressed gas storage
Liquid storage (cryogenic storage in vacuum
insulated or super insulated tank)
Line pack system ( allowing the pressure in the
transmission or distribution system to vary)
Underground storage ( in depleted oil and gas fields
or in aquifer systems)
Storage as metal hydrides.
You might also like
- Green Hydrogen PlantDocument6 pagesGreen Hydrogen PlantDilip50% (2)
- Hydrogen Basics - LecturaDocument21 pagesHydrogen Basics - LecturaFreddy GomezNo ratings yet
- Python Project SynopsisDocument30 pagesPython Project SynopsisAshish RoshanNo ratings yet
- Hydrogen Production, Storage and SaftyDocument9 pagesHydrogen Production, Storage and Saftyarunji76884No ratings yet
- Producing Technology of HydrogenDocument21 pagesProducing Technology of HydrogenOmer Utku Ozdemir100% (1)
- Hydrocarbons From Sustainable SourcesDocument6 pagesHydrocarbons From Sustainable Sourcesravi kansagaraNo ratings yet
- Dayalbagh Educational Institute: Faculty of EngineeringDocument14 pagesDayalbagh Educational Institute: Faculty of EngineeringShubham100% (2)
- Green HydrogenDocument3 pagesGreen HydrogenKhelidja DhmNo ratings yet
- Hydrogen ProductionDocument26 pagesHydrogen Productionsorincarmen88No ratings yet
- UntitledDocument11 pagesUntitledsahitNo ratings yet
- H2 ProductionDocument25 pagesH2 ProductionMohini SharmaNo ratings yet
- New Horizons For Hydrogen: Producing Hydrogen From Renewable ResourcesDocument8 pagesNew Horizons For Hydrogen: Producing Hydrogen From Renewable ResourcesBraulio BrunaudNo ratings yet
- 3.1. Steam ReformingDocument4 pages3.1. Steam ReformingSaju ShajuNo ratings yet
- Hydrogen PDFDocument8 pagesHydrogen PDFbrumbrumNo ratings yet
- Hydrogen Fuel CellDocument4 pagesHydrogen Fuel CellNayli SorfinaNo ratings yet
- NotesDocument15 pagesNotesSrinivasu ChennamsettiNo ratings yet
- Saminar Report: Hydrogen Is A Chemical Element With Symbol H and Atomic Number 1. With A StandardDocument25 pagesSaminar Report: Hydrogen Is A Chemical Element With Symbol H and Atomic Number 1. With A Standardved prakash raoNo ratings yet
- Water GasDocument2 pagesWater GasHammad AshrafNo ratings yet
- DetailsDocument19 pagesDetailsPrashanthMuraliNo ratings yet
- Hydrogen EnergyDocument12 pagesHydrogen Energysami almjanNo ratings yet
- Term Paper On Hydrogen Fuel A New HopeDocument15 pagesTerm Paper On Hydrogen Fuel A New Hopeprashant_cool_4uNo ratings yet
- Hidrogeno Combustible Del Futuro Parte 1Document6 pagesHidrogeno Combustible Del Futuro Parte 1Jose OlaNo ratings yet
- Hydrogen and EnergyDocument64 pagesHydrogen and EnergyFiveCent NickelNo ratings yet
- RER - Module - 3Document132 pagesRER - Module - 34JN20CS084 Rohit.DNo ratings yet
- H2 ProductionDocument43 pagesH2 Productionnouman khanNo ratings yet
- Vipppp4 BicakovaDocument14 pagesVipppp4 Bicakovahafeez khanNo ratings yet
- Hydrogen As Future Energy SourceDocument8 pagesHydrogen As Future Energy SourceWilliam ChangNo ratings yet
- Μικάλεφ Hyhdrogen Energy FacilitiesDocument30 pagesΜικάλεφ Hyhdrogen Energy FacilitiesKonstantinos TomprosNo ratings yet
- Brown GasDocument9 pagesBrown GasSagar Sachdeva100% (1)
- Hydrogen: The Future of Energy?Document12 pagesHydrogen: The Future of Energy?dsebdNo ratings yet
- The Performance of Ic Engine With (Diesel-Hydrogen) Dual FuelDocument7 pagesThe Performance of Ic Engine With (Diesel-Hydrogen) Dual FuelrassNo ratings yet
- Water SplittingDocument7 pagesWater SplittingRommel BalceNo ratings yet
- Electrolysis of WaterDocument5 pagesElectrolysis of WaterharishmechNo ratings yet
- HHO Generation System For WeldingDocument17 pagesHHO Generation System For WeldingAbhilash Koka100% (2)
- Hydrogen: The Perennial Source of EnergyDocument4 pagesHydrogen: The Perennial Source of Energyनिपुण कुमारNo ratings yet
- Hydrogen Fuel Basics - Clean EnergyDocument27 pagesHydrogen Fuel Basics - Clean EnergyKuldeepsingh ChandelNo ratings yet
- Hydrogen ProductionDocument15 pagesHydrogen ProductionsahitNo ratings yet
- Hydrogen As An Energy CarrierDocument13 pagesHydrogen As An Energy CarrierAyaz AhmadNo ratings yet
- Spark Machine: Mohamed Hassn & Abdallatif Mohamed & Kareem ZianDocument44 pagesSpark Machine: Mohamed Hassn & Abdallatif Mohamed & Kareem ZianMohamed HassanNo ratings yet
- B. B. ALE Department of Mechanical EngineeringDocument36 pagesB. B. ALE Department of Mechanical EngineeringRam Krishna SinghNo ratings yet
- Hydrogen: For XI STD ChemistryDocument139 pagesHydrogen: For XI STD ChemistryVed PrakashNo ratings yet
- Study of Hydrogen As An Industrial Gas: Presented By: Kamran Ashraf &Document39 pagesStudy of Hydrogen As An Industrial Gas: Presented By: Kamran Ashraf &Prabhu GovindNo ratings yet
- Entropy 22 01286 v2Document17 pagesEntropy 22 01286 v2scribduserme123No ratings yet
- Bio - HydrogenDocument18 pagesBio - HydrogenHarsh Vinay SinghNo ratings yet
- 5.hydrogen Energy NotesDocument8 pages5.hydrogen Energy Notesjayasruthyk6No ratings yet
- Two-Step Thermochemical Electrolysis An Approach For Green Hydrogen Production 21Document10 pagesTwo-Step Thermochemical Electrolysis An Approach For Green Hydrogen Production 21Abderrahim Najah ElidrissiNo ratings yet
- Green Hydrogen-Ali ElsayedDocument41 pagesGreen Hydrogen-Ali Elsayedali ahmedNo ratings yet
- Hydrogen ProductionDocument15 pagesHydrogen Production12mchc07No ratings yet
- 1 Hydrogen Productions and ChallengesDocument39 pages1 Hydrogen Productions and ChallengesFarhan TaufiqurrahmanNo ratings yet
- Fundamentals and Use of Hydrogen As A Fuel: ArticleDocument7 pagesFundamentals and Use of Hydrogen As A Fuel: ArticleJesseNo ratings yet
- Fundamentals and Use of Hydrogen As A Fuel: ArticleDocument7 pagesFundamentals and Use of Hydrogen As A Fuel: ArticleJesseNo ratings yet
- Daniela GOLEA, COZMA Lucian Ștefan - Ecological Fueling System For Vehicles Equipped With Combustion EnginesDocument12 pagesDaniela GOLEA, COZMA Lucian Ștefan - Ecological Fueling System For Vehicles Equipped With Combustion EnginesDaniela G GoleaNo ratings yet
- Steam Reforming Project ReportDocument2 pagesSteam Reforming Project ReportArundhati SharmaNo ratings yet
- Chapter 2Document7 pagesChapter 2pragati agrawalNo ratings yet
- 4-Hydrogen Production-19-Jul-2019Material - I - 19-Jul-2019 - Hydrogen - Properties - and - ProductionDocument44 pages4-Hydrogen Production-19-Jul-2019Material - I - 19-Jul-2019 - Hydrogen - Properties - and - ProductionPrabhu Sumanth NaiduNo ratings yet
- Electrolysis of WaterDocument8 pagesElectrolysis of Waterskyman05No ratings yet
- Hydrogen Production From Coal GasificatiDocument10 pagesHydrogen Production From Coal GasificatiProsperNo ratings yet
- The Hydrogen Economy PDFDocument15 pagesThe Hydrogen Economy PDFAbhinav GuptaNo ratings yet
- Digital Assignment - 1: Sanju Tom 19BME1232Document5 pagesDigital Assignment - 1: Sanju Tom 19BME1232sanju tomNo ratings yet
- Water Gas Shift Reaction: Research Developments and ApplicationsFrom EverandWater Gas Shift Reaction: Research Developments and ApplicationsNo ratings yet
- Failure Rate - TQMDocument8 pagesFailure Rate - TQMThanigaivel ANo ratings yet
- Thermionic ConverterDocument2 pagesThermionic ConverterThanigaivel A100% (1)
- CMM MQCDocument36 pagesCMM MQCThanigaivel ANo ratings yet
- CMM Working Priciple and Types and CalibrationDocument5 pagesCMM Working Priciple and Types and CalibrationThanigaivel ANo ratings yet
- Unit 4ppce PDFDocument88 pagesUnit 4ppce PDFThanigaivel A100% (1)
- Engineering Fundamentals of The Internal Combustion Engine - Willard W. PulkrabekDocument425 pagesEngineering Fundamentals of The Internal Combustion Engine - Willard W. Pulkrabekmi2jaca100% (2)
- 7 Gear Measurement P2Document12 pages7 Gear Measurement P2mahmoudNo ratings yet
- Economics 9 and 10Document4 pagesEconomics 9 and 10Thanigaivel ANo ratings yet
- Strength of Materials Lab PDFDocument34 pagesStrength of Materials Lab PDFThanigaivel ANo ratings yet