Professional Documents
Culture Documents
Pulido, Nathaniel Karl Enin T.
Uploaded by
Nathaniel Pulido0 ratings0% found this document useful (0 votes)
37 views1 pageThis document contains a student's answers to practice problems in biochemistry. It includes identifying solutions as acidic, basic, or neutral based on hydrogen and hydroxide ion concentrations. It also provides calculations to determine the concentrations of hydrogen and hydroxide ions in various solutions given their pH. Finally, it has the student complete a table with the pH, pOH, concentrations of H+ and OH-, and nature of 5 solutions.
Original Description:
sasasasasaasasasa
Original Title
Pulido,Nathaniel Karl Enin T.
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains a student's answers to practice problems in biochemistry. It includes identifying solutions as acidic, basic, or neutral based on hydrogen and hydroxide ion concentrations. It also provides calculations to determine the concentrations of hydrogen and hydroxide ions in various solutions given their pH. Finally, it has the student complete a table with the pH, pOH, concentrations of H+ and OH-, and nature of 5 solutions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
37 views1 pagePulido, Nathaniel Karl Enin T.
Uploaded by
Nathaniel PulidoThis document contains a student's answers to practice problems in biochemistry. It includes identifying solutions as acidic, basic, or neutral based on hydrogen and hydroxide ion concentrations. It also provides calculations to determine the concentrations of hydrogen and hydroxide ions in various solutions given their pH. Finally, it has the student complete a table with the pH, pOH, concentrations of H+ and OH-, and nature of 5 solutions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
Pulido, Nathaniel Karl Enin T.
November 2020
BSN 1-A
“BIOCHEMISTRY”
Scored exercises: Send Answers for record purposes to your Canvass
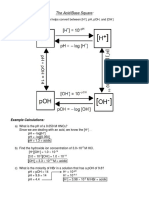
Identify the following as Acidic, Neutral or Basic
1. [H] = 2.45X10-12M 7. [OH]= 7.00X10-7M
-3
2. [H] = 1.44X10 M 8. pOH= 8.22
3. pH= 13.55 9. pOH = 6.25
4. pH=7.00 10. [H+]>[OH-]
5. pH= 1.77 11. [H+] <[OH-]
6. [OH]= 5.79X10-2 12. [H+] =[OH-]
Answers:
1. Basic 7. Basic
2. Acidic 8. Acidic
3. Basic 9. Basic
4. Neutral 10. Acidic
5. Acidic 11. Basic
6. Basic 12. Neutral
Calculate the concentrations of [H+] and [OH-] in the following solutions
1.Lemon juice, pH= 2.30 6. 0.79M HCL pH= .10
[H+]: 5.01 x 10-3 [H+]: 7.94 x 10-1
[OH-]: 2.0 x 10-12 [OH-]: 1.26 x 10-14
2.Carbonated water, pH= 3.00 7. 1.00M NaOH, pH= 14.00
[H+]: 1.0 x 10-3 [H+]: 1.0 x 10-14
[OH-]: 1.0 x 10-11 [OH-]: 1.0
3. Urine, pH= 6 8. Egg, pH = 7.80
[H+]: 1.0 x 10-6 [H+]: 1.58 x 10-8
[OH-]: 1.0 x 10-8 [OH-]: 6.31 x 10-7
4. Pure water, pH= 7
[H+]: 1.0 x 10-7
[OH-]: 1.0 x 10-7
5. Blood, pH= 7.4
[H+]: 3.98 x 10-8
[OH-]: 2.51 x 10-7
Complete the following table. Under the NATURE column, write Acidic, basic or neutral based on
your calculated answers
Solution pH pOH [H+] [OH-] NATURE
A 7.00 7.00 1.00 × 10-7 1.00 × 10-7 Neutral
B 2.25 11.75 5.62 × 10-3 1.78 × 10-12 Acidic
C 8.43 5.57 3.72 × 10-9 2.69 × 10-6 Basic
D 2.068 11.932 8.55X10-3 1.17 × 10-12 Acidic
E 5.243 8.757 5.71 × 10-6 1.75X10-9 Acidic
You might also like
- HYDROLYSIS OF SALTS AND PH OF BUFFER SOLUTIONSDocument13 pagesHYDROLYSIS OF SALTS AND PH OF BUFFER SOLUTIONSfadz607100% (2)
- Laboratory Report CHM 213 (Physical Chemistry) : 1. Muhammad Mirza Hizami Bin RajieiDocument6 pagesLaboratory Report CHM 213 (Physical Chemistry) : 1. Muhammad Mirza Hizami Bin RajieiMuhd Mirza Hizami100% (2)
- Pka PKB Acidos+bases Valores TablaDocument1 pagePka PKB Acidos+bases Valores TablaManuela Echevarria MontesNo ratings yet
- Neutralization: Prepared by Michigan Department of Environmental Quality Operator Training and Certification UnitDocument44 pagesNeutralization: Prepared by Michigan Department of Environmental Quality Operator Training and Certification UnitKhang TrầnNo ratings yet
- NCM 105A Exercise 1Document3 pagesNCM 105A Exercise 1Nathaniel PulidoNo ratings yet
- PH Problems: FormulasDocument4 pagesPH Problems: FormulasJoshua NaemonNo ratings yet
- Experiment No. 2 PH and BufferDocument7 pagesExperiment No. 2 PH and BufferTyn TynNo ratings yet
- Activity 1 PHDocument3 pagesActivity 1 PHPsalms Aubrey Domingo AcostaNo ratings yet
- 8.3 THE PH SCALEDocument26 pages8.3 THE PH SCALECLAIR OsiasNo ratings yet
- 8.3 The PH ScaleDocument22 pages8.3 The PH Scalelobna masadehNo ratings yet
- ACID, BASES AND PHDocument34 pagesACID, BASES AND PHDanica Alyssa CruzNo ratings yet
- Asam BasaDocument25 pagesAsam BasaFitriHdynNo ratings yet
- Acids Bases - Lesson 3 - PH & pOHDocument16 pagesAcids Bases - Lesson 3 - PH & pOHtausmanNo ratings yet
- Activity Phet Sim PH Relationships 1Document4 pagesActivity Phet Sim PH Relationships 1rebellchildersNo ratings yet
- Unit: Acids, Bases, and SolutionsDocument16 pagesUnit: Acids, Bases, and Solutionssana iqbalNo ratings yet
- Acid-Base EquilbriaDocument67 pagesAcid-Base EquilbriaKaela Beatrice Sy LatoNo ratings yet
- Modul 7 Kimdas Mardiah - 155Document11 pagesModul 7 Kimdas Mardiah - 155Mardiah DiahNo ratings yet
- WTRDocument51 pagesWTRsizarNo ratings yet
- Worksheet 5. Acids, Bases and BuffersDocument1 pageWorksheet 5. Acids, Bases and Buffersmichael.obachNo ratings yet
- Latihan KesetimbanganDocument61 pagesLatihan KesetimbanganHazelnut ChocoNo ratings yet
- Question and DiscussionDocument5 pagesQuestion and DiscussionNoviana Sri RahayuNo ratings yet
- Lectures For College of Biotechnology. Analytical ChemistryDocument8 pagesLectures For College of Biotechnology. Analytical ChemistryB1 عبدالله عبدالامير هاديNo ratings yet
- Acids and Bases Notes OutlineDocument12 pagesAcids and Bases Notes OutlineSidharth RajagopalanNo ratings yet
- Concept of PHDocument18 pagesConcept of PHJohn mark PunayNo ratings yet
- Expt 1 PH MeasurementDocument33 pagesExpt 1 PH MeasurementColene MoresNo ratings yet
- Acid Base Assignment: Sch4U, Chemistry Ribba Pathan July 1, 2017Document4 pagesAcid Base Assignment: Sch4U, Chemistry Ribba Pathan July 1, 2017Namdeo JadhavNo ratings yet
- Informe PH y Ph-MetriaDocument9 pagesInforme PH y Ph-MetriaMafe CadavidNo ratings yet
- Workbook Answers Acids BasesDocument21 pagesWorkbook Answers Acids BasesGalah NasserNo ratings yet
- Acid-Base Unit Review Questions Answer KeyDocument3 pagesAcid-Base Unit Review Questions Answer KeySamia KabirNo ratings yet
- Lecture 8. PH and Dissociation of Water-MirDocument29 pagesLecture 8. PH and Dissociation of Water-MirMohammedNo ratings yet
- Acids and Bases I. in 3-5 Sentences, Briefly Answer The Following Review Questions 1Document5 pagesAcids and Bases I. in 3-5 Sentences, Briefly Answer The Following Review Questions 1Patricia Bianca BunagNo ratings yet
- Name: Bunag, Patricia Bianca S. Course: LJPS01 Acids and Bases I. in 3-5 Sentences, Briefly Answer The Following Review Questions 1Document5 pagesName: Bunag, Patricia Bianca S. Course: LJPS01 Acids and Bases I. in 3-5 Sentences, Briefly Answer The Following Review Questions 1Patricia Bianca BunagNo ratings yet
- U08 Notes Part2 PHDocument22 pagesU08 Notes Part2 PHapi-546066323No ratings yet
- Buffer SolutionsDocument21 pagesBuffer SolutionsRizka Nur FaridaNo ratings yet
- Teacher Guide 10Document26 pagesTeacher Guide 10Raza AbbasNo ratings yet
- Worksheet 2 PH Measurement and Buffer PreparationDocument15 pagesWorksheet 2 PH Measurement and Buffer PreparationAkeysha CarreonNo ratings yet
- Activity Phet Sim PH RelationshipsDocument3 pagesActivity Phet Sim PH RelationshipsMarini Anggytha Fivani SianiparNo ratings yet
- M10L2 NotesDocument1 pageM10L2 NotesEmilyNo ratings yet
- Monoprotic Acid-Base Equilibria: 1. Strong Acids & Bases: Dilemma in Calculating The PH of Strong Acids & BasesDocument15 pagesMonoprotic Acid-Base Equilibria: 1. Strong Acids & Bases: Dilemma in Calculating The PH of Strong Acids & BasesSkygazerNo ratings yet
- GenChem2 Q4 MELC 7-9 Week-5Document7 pagesGenChem2 Q4 MELC 7-9 Week-5BSED FIL 1- Ashley Romarie A. LactaotaoNo ratings yet
- Chem12 SM 08 5Document9 pagesChem12 SM 08 5Tithi ChoksiNo ratings yet
- Acid Bases and BuffersDocument6 pagesAcid Bases and BuffersKathryne May JinonNo ratings yet
- KW of Water Part IIIDocument16 pagesKW of Water Part IIIMenalqueNo ratings yet
- GenChem2 - Q4 - M2 - Acid Base Equilibria and Buffer SolutionsDocument5 pagesGenChem2 - Q4 - M2 - Acid Base Equilibria and Buffer SolutionsАртем МонтерейNo ratings yet
- Acid Base 2006Document24 pagesAcid Base 2006Vina SoumokilNo ratings yet
- CHM 213 - Exp 5Document9 pagesCHM 213 - Exp 5hafiqahNo ratings yet
- Tugas Kimia FarmasiDocument4 pagesTugas Kimia FarmasiLtifaNo ratings yet
- chm101 Lab Acids and Bases Grading RubricDocument7 pageschm101 Lab Acids and Bases Grading RubricRealina GunabeNo ratings yet
- Lecture 5 Acids and BasesDocument39 pagesLecture 5 Acids and BasesAllen SiaNo ratings yet
- Wrd-Ot-Neutralization 445273 7Document44 pagesWrd-Ot-Neutralization 445273 7Sheik Abdullah BakrudeenNo ratings yet
- 8.4 - 1 Strong and Weak Acids (H+) and PH CalculationsDocument4 pages8.4 - 1 Strong and Weak Acids (H+) and PH Calculations2018dgscmtNo ratings yet
- PH - A Measure of Hydrogen Ion Activity: PH H or PH HODocument4 pagesPH - A Measure of Hydrogen Ion Activity: PH H or PH HOAdrien OfthestoneNo ratings yet
- Define PH and Use The Hydrogen or Hydroxide Ion Concentrations To Calculate The PH of A SolutionDocument1 pageDefine PH and Use The Hydrogen or Hydroxide Ion Concentrations To Calculate The PH of A Solutionapi-87739323No ratings yet
- 14 Acids, Bases and SaltsDocument105 pages14 Acids, Bases and Saltsmika yzobelNo ratings yet
- NCM 103 Final ExamDocument5 pagesNCM 103 Final ExamRichmond LacadenNo ratings yet
- Chapter 19 Acids and Bases PPT Glembocki 2017Document32 pagesChapter 19 Acids and Bases PPT Glembocki 2017Master NistroNo ratings yet
- CB03.11.18.ACID BASE Biological SystemsDocument6 pagesCB03.11.18.ACID BASE Biological SystemsLavanyadNo ratings yet
- Chapter 15Document14 pagesChapter 15Ahmed AlbagerNo ratings yet
- Lesson 14.4 PH of SolutionDocument4 pagesLesson 14.4 PH of SolutionKhate AlNo ratings yet
- 2 Forms &routes of Drug AdminDocument38 pages2 Forms &routes of Drug AdminNathaniel PulidoNo ratings yet
- Understanding Drug Labels: Belen G. de Los Trinos, RN, RM, USRN, MANDocument35 pagesUnderstanding Drug Labels: Belen G. de Los Trinos, RN, RM, USRN, MANNathaniel PulidoNo ratings yet
- Pharma RLE OverviewDocument10 pagesPharma RLE OverviewNathaniel PulidoNo ratings yet
- Partograph EditedDocument54 pagesPartograph EditedNathaniel PulidoNo ratings yet
- Medications Practice Problems (With Answers) : Sheet 1Document11 pagesMedications Practice Problems (With Answers) : Sheet 1Nathaniel PulidoNo ratings yet
- Laboratory Exercise No. 1Document5 pagesLaboratory Exercise No. 1Nathaniel PulidoNo ratings yet
- 10 Rights in Drug AdministrationDocument25 pages10 Rights in Drug AdministrationNathaniel PulidoNo ratings yet
- RLE Generic Reqts Formats and RubricsDocument9 pagesRLE Generic Reqts Formats and RubricsNathaniel PulidoNo ratings yet
- Name of Recipe: English Muffin Pizza Important Notes: (Deficiency Symptoms or Toxicity Preservation Methods)Document1 pageName of Recipe: English Muffin Pizza Important Notes: (Deficiency Symptoms or Toxicity Preservation Methods)Nathaniel PulidoNo ratings yet
- Asynchronous ActivityDocument2 pagesAsynchronous ActivityNathaniel PulidoNo ratings yet