Professional Documents
Culture Documents
Worksheet 5. Acids, Bases and Buffers
Uploaded by
michael.obach0 ratings0% found this document useful (0 votes)
12 views1 pageCopyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
12 views1 pageWorksheet 5. Acids, Bases and Buffers
Uploaded by
michael.obachCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
Arellano University
1st Semester, 2022-2023
Worksheet 5. Acids, Bases and Buffers
Name ____________________________________
I. Complete the table
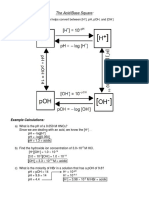
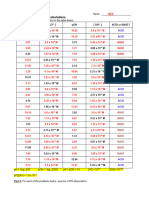
pH [ H3O+ ] or [H+] pOH [ OH– ] ACID or BASE?
11.86 2.14 7.24 x 10-3 M Base
1.38 x 10-12 M
10.6
3.40 3.98 x 10 -4 M 2.31 x 10–11 M Acid
10.91 1.23 x 10-11 M 8.13 x 10-4 M Base
3.09
8.87
5.13 7.49 x 10–6 M 1.35 x 10-9 M Acid
II. Determine the conjugate acid for the following bases:
1. OH- = OH- + H+ = H2O
2. HSO4-1 = HSO4 + H+ = H2SO4
3. ClO4-1 = HCIO4
III. Determine the conjugate base for the following acids:
1. HPO4-2 = PO4-3
2. HCO3-1 = CO3 -2
3. HBrO2 = BrO2-
1. Calculate the pH of an acetic acid (HAc) buffer with [H +] = 0.15 and [Ac-] = 0.18 if the
pka of acetic acid is 4.75.
b. if 0.01M of HCl is added to this solution, what will be the new pH?
c. if 0.01M of NaOH is added to this solution, what will be the new pH?
d. Is this a good buffer? Justify your answer.
You might also like
- NCM 103 Final ExamDocument5 pagesNCM 103 Final ExamRichmond LacadenNo ratings yet
- Combined PH WorksheetsDocument9 pagesCombined PH WorksheetsNeen NaazNo ratings yet
- Laboratory Report CHM 213 (Physical Chemistry) : 1. Muhammad Mirza Hizami Bin RajieiDocument6 pagesLaboratory Report CHM 213 (Physical Chemistry) : 1. Muhammad Mirza Hizami Bin RajieiMuhd Mirza Hizami100% (2)
- Activity 1 PHDocument3 pagesActivity 1 PHPsalms Aubrey Domingo AcostaNo ratings yet
- Practice Exercise 5.1: Joana Lyn L. Torres BS Psych 1-BDocument5 pagesPractice Exercise 5.1: Joana Lyn L. Torres BS Psych 1-BJoana Lyn Torres0% (1)
- CHEM 123 Sample Exam 1 Multiple Choice and Short Answer QuestionsDocument7 pagesCHEM 123 Sample Exam 1 Multiple Choice and Short Answer QuestionsRoberto BeltNo ratings yet
- Acid-Base Chemistry. Extra Practice Problems General Types/Groups of ProblemsDocument13 pagesAcid-Base Chemistry. Extra Practice Problems General Types/Groups of ProblemsYeabisraNo ratings yet
- General Chemistry II Exam 3 Multiple Choice ReviewDocument6 pagesGeneral Chemistry II Exam 3 Multiple Choice ReviewAnonymous rFIshYyNo ratings yet
- PH Worksheet SolutionsDocument3 pagesPH Worksheet Solutionsxdiep10No ratings yet
- PH Problems: FormulasDocument4 pagesPH Problems: FormulasJoshua NaemonNo ratings yet
- Honors Chemistry QuizDocument4 pagesHonors Chemistry QuizmariaNo ratings yet
- Acidbase L207Document5 pagesAcidbase L207silvergold888No ratings yet
- Acids and Bases Key ConceptsDocument8 pagesAcids and Bases Key ConceptsAivan NovillaNo ratings yet
- Chemistry 101 Solutions for Acids and Bases AssignmentDocument3 pagesChemistry 101 Solutions for Acids and Bases AssignmentmikecostantiniNo ratings yet
- PH Worksheet KeyDocument2 pagesPH Worksheet KeyLane ButterworthNo ratings yet
- Pulido, Nathaniel Karl Enin T.Document1 pagePulido, Nathaniel Karl Enin T.Nathaniel PulidoNo ratings yet
- PreAP Chemistry Unit 16 Review: Acids, Bases, and pH CalculationsDocument2 pagesPreAP Chemistry Unit 16 Review: Acids, Bases, and pH CalculationsJoshua Dedmon-StudentNo ratings yet
- GenChem2 - Q4 - M2 - Acid Base Equilibria and Buffer SolutionsDocument5 pagesGenChem2 - Q4 - M2 - Acid Base Equilibria and Buffer SolutionsАртем МонтерейNo ratings yet
- Chap 19Document4 pagesChap 19Jimini KimNo ratings yet
- Acid-Base Unit Review QuestionsDocument4 pagesAcid-Base Unit Review QuestionsSamia KabirNo ratings yet
- Acid-Base Unit Review Questions Answer KeyDocument3 pagesAcid-Base Unit Review Questions Answer KeySamia KabirNo ratings yet
- NS1Lec - Module 5 - NacionalesDocument5 pagesNS1Lec - Module 5 - NacionalesWindere Marie NacionalesNo ratings yet
- Worksheet 5. Aqueous Equilibrium Problems Simple EquilibriaDocument3 pagesWorksheet 5. Aqueous Equilibrium Problems Simple EquilibriaJohnHenryYambaoNo ratings yet
- Acids Bases and PH WorksheetDocument2 pagesAcids Bases and PH WorksheetrabiaNo ratings yet
- AP Unit9 Worksheet AnswersDocument5 pagesAP Unit9 Worksheet AnswersAAVANINo ratings yet
- Asam BasaDocument25 pagesAsam BasaFitriHdynNo ratings yet
- PH and PohDocument2 pagesPH and Pohapi-483662721No ratings yet
- AB Salts WKST KeyDocument10 pagesAB Salts WKST Keyashay koradiaNo ratings yet
- Latihan KesetimbanganDocument61 pagesLatihan KesetimbanganHazelnut ChocoNo ratings yet
- Question and DiscussionDocument5 pagesQuestion and DiscussionNoviana Sri RahayuNo ratings yet
- Buffer SolutionsDocument21 pagesBuffer SolutionsRizka Nur FaridaNo ratings yet
- Chapter 3 Answers 2019-2020Document11 pagesChapter 3 Answers 2019-2020Nuraina NabihahNo ratings yet
- GenChem2 Q4 MELC 7-9 Week-5Document7 pagesGenChem2 Q4 MELC 7-9 Week-5BSED FIL 1- Ashley Romarie A. LactaotaoNo ratings yet
- I. Multiple Choice. Write The Best Answer From The Following ChoicesDocument5 pagesI. Multiple Choice. Write The Best Answer From The Following ChoicesDoom Refuge100% (1)
- Tpoic 3Document12 pagesTpoic 3Marvin EusebioNo ratings yet
- AB Titration WSDocument6 pagesAB Titration WSiwinthushaaNo ratings yet
- Ch. 17 - Practice Problems With Buffers - ANSWERSDocument4 pagesCh. 17 - Practice Problems With Buffers - ANSWERSKavi BhagatNo ratings yet
- Acid Base Eqm. Worksheets UPDATED 2022Document24 pagesAcid Base Eqm. Worksheets UPDATED 2022Sara MolinaroNo ratings yet
- اسیدها و بازهاDocument31 pagesاسیدها و بازهاapi-37062900% (1)
- Acid Base Problem Set 1Document3 pagesAcid Base Problem Set 1Тахмина ЗульфугароваNo ratings yet
- Lesson Plan Acid N BaseDocument3 pagesLesson Plan Acid N BasedediyanNo ratings yet
- Acid Base Concepts (Quiz With Answers)Document12 pagesAcid Base Concepts (Quiz With Answers)heylinssNo ratings yet
- Work Sheet PH and pOH AnswersDocument3 pagesWork Sheet PH and pOH Answersparamabc27No ratings yet
- Chemistry: PH and pOH Calculations: Part 1: Fill in The Missing Information in The Table BelowDocument6 pagesChemistry: PH and pOH Calculations: Part 1: Fill in The Missing Information in The Table BelowCaryl Ann C. SernadillaNo ratings yet
- Chem 46 PS1Document1 pageChem 46 PS1sjhedmrblackNo ratings yet
- PH WsDocument1 pagePH WsTutor AcademyNo ratings yet
- Chem12 4 3 Worksheet Student PDFDocument1 pageChem12 4 3 Worksheet Student PDFabmacphailNo ratings yet
- Topic 4 QuizDocument4 pagesTopic 4 QuizGraceljaneNo ratings yet
- Chapter 15 PracticeDocument2 pagesChapter 15 PracticeGNCDWNo ratings yet
- 05 - The Chemistry of Acids and Bases Complete - RevisedDocument63 pages05 - The Chemistry of Acids and Bases Complete - RevisedKabesang TalesNo ratings yet
- Acids and Bases I. in 3-5 Sentences, Briefly Answer The Following Review Questions 1Document5 pagesAcids and Bases I. in 3-5 Sentences, Briefly Answer The Following Review Questions 1Patricia Bianca BunagNo ratings yet
- Buffer ActivityDocument2 pagesBuffer ActivityJainee Chen JavillonarNo ratings yet
- CHM 213 - Exp 5Document9 pagesCHM 213 - Exp 5hafiqahNo ratings yet
- Name: Bunag, Patricia Bianca S. Course: LJPS01 Acids and Bases I. in 3-5 Sentences, Briefly Answer The Following Review Questions 1Document5 pagesName: Bunag, Patricia Bianca S. Course: LJPS01 Acids and Bases I. in 3-5 Sentences, Briefly Answer The Following Review Questions 1Patricia Bianca BunagNo ratings yet
- Packet Chapter 15 - Aqueous Equilibrium 2010Document46 pagesPacket Chapter 15 - Aqueous Equilibrium 2010Jeffrey ManNo ratings yet
- Acid Base Intro Powerpoint 2020Document35 pagesAcid Base Intro Powerpoint 2020JulesNo ratings yet
- Gen Chem II EX 4 Practice Problems Sp08Document6 pagesGen Chem II EX 4 Practice Problems Sp08Wong Chee KheonNo ratings yet
- Carbonic Hydronium Conc - PDFDocument2 pagesCarbonic Hydronium Conc - PDFbencleeseNo ratings yet
- Which is not a characteristic property of acidsDocument12 pagesWhich is not a characteristic property of acidsIsisahNo ratings yet
- Recent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumFrom EverandRecent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumW. D. OllisNo ratings yet