Professional Documents
Culture Documents
Paschoal Et Al. (2020) - AABC
Uploaded by
Lucas PaschoalOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Paschoal Et Al. (2020) - AABC
Uploaded by
Lucas PaschoalCopyright:
Available Formats
An Acad Bras Cienc (2020) 92(Suppl. 2): e20180811 DOI 10.
1590/0001-3765202020180811
Anais da Academia Brasileira de Ciências | Annals of the Brazilian Academy of Sciences
Printed ISSN 0001-3765 I Online ISSN 1678-2690
www.scielo.br/aabc | www.fb.com/aabcjournal
BIOLOGICAL SCIENCES
Massive mortality of the giant freshwater
mussel Anodontites trapesialis (Lamarck,
1819) (Bivalvia: Mycetopodidae) during a
severe drought in a Neotropical reservoir
LUCAS R.P. PASCHOAL, DOUGLAS P. ANDRADE, DANIEL M. PIMPÃO,
SANTIAGO TORRES & GUSTAVO DARRIGRAN
Abstract: In 2012, a severe drought struck the southeastern of Brazil compromising the
Paraná River Basin reservoirs. Here, we described how this climatic event promoted
a massive mortality of the giant freshwater mussel Anodontites trapesialis in Furnas
reservoir and reported the consequences of this phenomenon. In November 2012, three
quarters of 100 m2 were sampled in this reservoir, where 812 dead shells of A. trapesialis
were analyzed and measured_ (33 ˫ 133 mm). The species showed an aggregated
distribution with high density (X : 1.0 - 5.5 ind/m2). Despite the massive mortality detected
in field, it was possible to find living specimens in a small channel in the studied area,
allowing the species to survive the water level fluctuations. Large adult individuals (100
˫ 124 mm) were more affected by drought than juveniles, accounting for about 90% of
the dead mussels analyzed. Two years after the massive mortality event, water level
was not reestablished and a terrestrial succession (with elevations in the concentration
of organic matter and calcium in sediment) was observed in the studied area. We
verified that the damming associated with extreme climatic events affect negatively the
populations of A. trapesialis and should be faced as a conservationist problem.
Key words: Unionida, die-off, hydric stress, conservation, Mollusca.
INTRODUCTION farming (Graf & Cummings 2007, Pereira et al.
2014, Torres et al. 2018). This is due to the fact
The giant freshwater mussel Anodontites that their larvae (lasidium type) are capable to
trapesialis (Lamarck, 1819) is a functional use non-native fish species as hosts, as is the
simultaneous hermaphrodite bivalve with a case of the tilapia Oreochromis spp. (Guardia-
trapezoid shell, with records of some specimens Felipi & Silva-Souza 2008, Callil et al. 2012).
reaching over than 200 mm (Simone 1994, Callil However, when this species inhabits reservoirs it
& Mansur 2007). It is the most widely distributed suffers from certain environmental restrictions
bivalve in the South America basins, and shows due to damming, which significantly affects
a wide geographical distribution in Americas, its population structure (L.R.P. Paschoal & D.P.
occurring from some Central America basins to Andrade, unpublished data).
Argentinean Patagonian basins. This species, Reservoirs are artificial man-made
which occurs naturally in lotic environments environments used for multiple purposes,
has been commonly found in artificial lakes such as: leisure, energy production and public
and reservoirs, especially in areas used for fish supply. Brazil is the country with the largest
An Acad Bras Cienc (2020) 92(Suppl. 2)
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
number of reservoirs (N = 393) in the world River Basin (southeastern of Brazil) due to a
(FAO 2016). In general, they are considered as prolonged drought. In 2012, a severe drought
intermediate type water bodies, since they was recorded for the Southeast region of
have a retention time of two to forty days. Brazil, promoting decreases in water levels in
The upstream area of these environments has certain reservoirs of this region up to 17 meters
lentic characteristics, while the downstream below the usual average for the same period
areas have lotic conditions (Henry 1999, Brasil (ANA 2017, ONS 2017). Considering this scenario,
2012). In these environments, certain natural the present study verified how the decrease
physical-hydrological phenomena (added or of the water level in a Neotropical reservoir
not by anthropic impacts) occur irregularly in promoted by drought and damming, caused
time and space, promoting instability in benthic a massive mortality of the giant freshwater
communities that live in these areas. The main mussel A. trapesialis. In addition, we pointed
natural limiting phenomena (factors) are the ecological and conservation implications of this
deposition of particulate matter, the action of phenomenon.
waves on the margins and water column level
fluctuations (Agostinho et al. 1992, Andrade et al.
2012, Paschoal et al. 2015). MATERIALS AND METHODS
The decrease in water level and the exposure Study site
to air caused by droughts are considered the main The reservoir of the Furnas Hydroelectric Power
factors responsible for desiccation in bivalves Station (HPS) is composed mainly by the Grande
(Collas et al. 2014, Paschoal et al. 2015). Some and Sapucaí rivers (upper Paraná Basin) (Fig. 1).
species of freshwater mollusks are capable of It is the largest reservoir in the state of Minas
surviving to desiccation by hibernating and/or Gerais (flooded area:1,440 km2) and one of the
migrating to deeper or more humid aquatic zones most important in Brazil, extending throughout
(Darrigran & Lopez-Armengol 1998, Darrigran & 36 municipalities. The reservoir is used for
Lagreca 2005). However, several studies have energy production, as well as a water reserve,
reported a massive mortality of bivalves caused a recreational place and source of income for
by desiccation, when high density and biomass many families that live from fishing, and also
of individuals were transported and stranded on directly and indirectly harbors great diversity of
the coastal areas due to environmental changes wildlife (Paschoal et al. 2012).
caused by extreme climatic events (e.g. Ilarri et The present study was conducted at the
al. 2011, Sousa et al. 2012, 2018, Paschoal et al. margins of a portion of the Sapucaí River (20° 58’
2015, Vaughn et al. 2015). In these studies, the 23” S, 46° 07’ 08” W), inserted in the Furnas HPS
authors correlated this situation mainly to the reservoir, municipality of Carmo do Rio Claro,
low capacity of locomotion of these animals, Minas Gerais state (southeastern of Brazil) (Figs.
added to temporal variability of the physical 1 and 2). This area is characterized by high water
conditions of these transitional environments. column fluctuation levels throughout the year,
In recent years, severe droughts have clay-sandy sediment, pebbles and boulders in its
dramatically altered the aquatic environments margins, and predominance of the macrophyte
of Brazil (Nazareno & Laurance 2015). Melo et Brachiaria sp. near the margin and Eichhornia
al. (2016) verified that the water supply was azurea (Swartz) Kunth ten meters away from the
much compromised in reservoirs of the Paraná margin (Paschoal et al. 2015).
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 2 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
Figure 1. Map showing sampling site location at Furnas HPS reservoir (state of Minas Gerais, southeastern Brazil).
The water column levels and accumulated Data sampling
precipitation patterns during the period from Sampling was performed on November 2nd, 2012.
January 2000 to December 2015 were verified The collection and analysis permissions were
using data from ANA (2017) and ONS (2017). granted by MMA/ICMBio/SISBIO (license 36210-
Mean monthly values of these variables were 1). Twelve living bivalves were collected in a
computed, showing that from June to October small channel (i.e. original river channel before
the water column level of the reservoir tends impoundment) near to the sampled area (Figs.
to decline due to low precipitation levels in the 2b and 4) and transported to the laboratory.
previous months (May to September), until it These mussels were identified according to
reaches its minimum level in November (Fig. 3a). Simone (1994, 2006) and had its shell length
However, it was possible to observe that in 2012, (L), height (H) and width (Wi) measured (Fig.
December was the month with the lowest water S1 - Supplementary Material). The wet weight
column level due to a severe drought occurred (We) of each individual was obtained with an
in the Southeastern region of Brazil (Fig. 3b). The analytical scale (± 0.001 g), after being dried with
storage capacity (volume of the flooded area) absorbent paper. Subsequently, these animals
of the Furnas HPS reservoir fell below 13% of were euthanized by freezing (-20 °C / 30 min.)
its total capacity in December, and during the and preserved in 70% ethanol. These bivalves
months of November and December showed were deposited at the Museu de Zoologia da
the water column level below five meters (Fig. Universidade de São Paulo (MZSP), under the
3b), far from the average recorded for the last number 109106.
15 years for the region - 9.2 to 10.5 meters (Fig. In field, we analyzed three areas along the
3a). The present study was only possible to be margin of the Sapucaí River channel, each one
performed in the present condition (i.e. drought comprising a quarter of 100 m 2 (subdivided
and lower water column levels), since such into 100 quadrats of 1 m2). These quarters were
areas are inaccessible when the reservoir is in distant 40 meters from each other, and the
its normal hydrological conditions (Fig. 2). right side of each quarter was the closest to the
channel margin. There were no evidences for a
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 3 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
Figure 2. Variation of the water column in sampling site at Furnas HPS reservoir (Sapucaí River, state of Minas
Gerais). a. 2011 - Flooded area (typical pattern). b. 2012 - Exposed area due to a severe drought. c. 2014 - Terrestrial
succession after two years of the massive mortality of Anodontites trapesialis. a-b. Modified from Paschoal et al.
(2015).
strong depth gradient along the sampled areas. Data analysis
Also, these areas were exposed to sunlight for Allometric equations of Y = aX b type were
a considerable amount of time. This procedure adjusted for the 12 living specimens, having as
allowed to estimate the population density (ind./ independent variable (X) the shell length (L) and
m2), biometric values of the shells and spatial relating it to the other body dimensions of the
distribution of A. trapesialis in the studied area. animal (dependent variables, Y) (Huxley 1950).
For each sampled quarter, all dead shells (i.e. Values of the allometric constant (b) were tested
without soft parts) inside the quadrats showing using the t test, as H0:b = 1 (or 3 in the case of
the two valves were identified, counted and weight), and used to determine the growth
measured (L, H and Wi) with an analogic caliper patterns of a specific body part in relation to L.
(0.05 mm) (Figs. 4 and S2). Shell fragments and Subsequently, the equations were linearized (Y
buried individuals that could not be identified = a * X - b).
were disregarded and excluded from analysis. The distribution pattern of A. trapesialis
The first quarter (I) was inserted in a grassy was determined using the Morisita index (IM),
area (with predominance of Zoysia sp.) near to being random when I M = 1, aggregate when
the river channel, with high content of organic IM > 1, and uniform when IM < 1 (Krebs 1989).
matter in soil (61 g/kg) and clayey sediment Analyses of variance (ANOVA’s) associated with
(Fig. 4). The other quarters (II and III) showed Tukey multiple comparison tests were used to
longer exposure to sunlight due to lower verify possible differences between the mean
depth in relation to the Quarter I, with a higher values of density and biometric variables of A.
proportion of silt in its sediment composition trapesialis dead shells between the quarters.
and no grasses in their soil, indicating a lower
amount of organic matter (20 g/kg) (L.R.P.
Paschoal, personal data). RESULTS
On November 2nd 2014, two years after the
The mean (± standard deviation) shell length
massive mortality event detected in Furnas
(L) of the 12 living specimens of A. trapesialis
HPS reservoir, a new sampling (using the
was 121.1 ± 6.0 mm, while the shell height (H)
same methodology) was performed to verify a
and width (Wi) were 61.3 ± 4.1 and 37 ± 4.1 mm,
recovery (or not) of the number of individuals in
respectively. The mean weight (We) of these
this population.
mussels was 96.32 ± 15.17 g. The smallest
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 4 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
Figure 3. a. Accumulated precipitation and water column mean values from 2000 to 2015, and b. absolute values
from 2012 for the Sapucaí River inserted at Furnas HPS reservoir.
individual analyzed had 113.5 mm L, 59.4 mm H, estimated by extrapolating a linear regression,
37.5 mm Wi and 108.34 g We, while the largest were: 43.45, 7.21 and 12.07 kg/quarter, for Quarter
animal had 133.3 mm L, 67.6 mm H, 42.6 mm Wi 1 to 3, respectively.
and 119.89 g We. Table I summarizes the linear We observed the presence of Nile tilapia
regressions of the morphometric data and Oreochromis niloticus (Linnaeus, 1758) nests near
shows allometric patterns for the analyzed body to the analyzed quarters, especially in the area
portions. It is noted that only the relationship H of Quarter I (Fig. S3). The highest shell length (L)
vs. L showed positive allometry (Table I). frequencies of dead mussels measured during
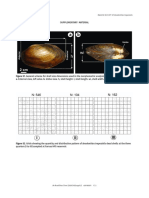
In field, 812 dead shells of A. trapesialis the study were recorded for the size classes
were analyzed and measured (Figs. 4 and between 100 and 124 mm (approximately 87% of
S2). Statistically significant differences were the sampled dead shells), with a modal peak at
registered in the total number of individuals the size class 110 ˫ 114mm. Few specimens were
obtained in the three quarters (F = 82.05, p < found in the lower size classes. The smallest
0.001), as well as in the mean size of the body dead mussel analyzed in field had 30.0 mm L,
structures measured in mussels sampled in 12.0 mm H and 9.8 mm Wi, while the largest had
these quarters (F = 14.89, p < 0.001). Quarter I 133.0 mm L, 67.7 mm H and 42.2 mm Wi (Fig. 5).
showed higher abundance (N = 546) and density In 2014, the second field survey cannot be
_
(X = 5.52 ind/m2), besides having animals with performed due to the abundance of shrubs and
_
greater body proportions in its composition (X = erect macrophytes of the genera Polygonum
111 mm), when compared to the other quarters and Brachiaria (Fig. S4), indicating that area is
_
(X = 106 and 109 mm). In addition, the highest in an initial process of terrestrial succession
absolute density (22 ind/m2) recorded in this (Fig. 2c). The water level was not recovered;
study was established in this quarter. The consequently, the mussels do not come back
population of A. trapesialis that inhabited the to this area. Besides that, only shell fragments
Sapucaí River showed an aggregated distribution were recorded among this vegetation.
(IM > 1) along the sampled area, i.e. all quarters
(Fig. S2 and Table II). The biomass values
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 5 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
Figure 4. a-b. Massive mortality of Anodontites trapesialis with great accumulation of dead shells at Furnas HPS
reservoir. Black arrows indicate the channel where living specimens were collected. Note the dry cracked soil
during drought.
DISCUSSION 2018, Bódis et al. 2014, Paschoal et al. 2015,
Vaughn et al. 2015).
In 2012, a severe drought in Brazilian southeast Despite the massive mortality verified in
region promoted a sudden change and decrease field during the drought, it was possible to find
of the water levels of the reservoir analyzed living specimens of A. trapesialis in a small
here. These events have caused a great negative channel in the studied area. This shows how
impact on the population of Anodontites the species is capable to adapt to different
trapesialis, evidenced by the expressive number environmental conditions and to survive even in
of mussels killed by desiccation, i.e. massive hydric stress events, as pointed out by Pereira et
mortality. Pilger & Gido (2012) demonstrated al. (2014). As observed here, there are indications
that freshwater mussels were heavily affected that the plasticity of A. trapesialis population
by rapidly receding water levels. The giant from Furnas HPS reservoir has allowed the
mussels could not move in the same rhythm as species to survive the water levels fluctuations,
the rapid decrease of the water column level which in natural environments does not occur
of the Furnas reservoir, being exposed to the so quickly. Callil & Mansur (2007) and Callil et
environment and susceptible to desiccation al. (2018) confirmed that the reproductive cycle
and predators. Cândido & Romero (2006, 2007) of A. trapesialis, as well as the energy allocation
demonstrated that A. trapesialis shows slow for growth of this mussel in the Pantanal
rates of burrowing, digging and displacement/ wetlands are directly related to the hydrological
locomotion. Thus, it is possible that these cycle imposed by flood pulses. In this way,
mussels have stranded on the sediment when future studies are necessary to verify if the
exposed to drought, promoting a significant population analyzed in this reservoir maintains
mortality in this population. This pattern is the pattern of body increment and reproductive
similar to that recorded for other unionoid and peak influenced by higher water levels or if it
cirenid bivalves exposed to extreme climatic is capable to reproduce and maintain constant
events (see Ilarri et al. 2011, Sousa et al. 2012, sizes over time, as registered for Corbicula
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 6 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
Table I. Relationships between shell length (L) and morphometric variables of Anodontites trapesialis living
specimens.
t
Linear regression N r² A
(b = 1 or 3)
H = 0.54L - 4.27 12 0.63 3.48* +
Wi = 0.31L + 0.37 12 0.71 10.13* -
We = 1.64L - 103.08 12 0.54 1.21* -
, statistically significant at p < 0.001. A, allometry. H, shell height. r2, coefficient of determination. We, shell weight. Wi, shell
*
width.
fluminea (Müller, 1774) in the same reservoir by Few studies evaluated the population density of
Paschoal et al. (2015). Anodontites mussels in Neotropical reservoirs.
The population of A. trapesialis analyzed Nascimento-Filho et al. (2014) recorded a density
invests energy for longitudinal shell growth. up to 1 ind/m2 for A. trapesialis in the Serrinha
This is probably related to the growth of the reservoir (Pernambuco state, northeastern
foot and demibranches as the shell increases. Brazil). Henry & Simão (1986) registered a density
A large foot would optimize the time spent in of up to 0.02 ind/m2 for the congener A. trapezeus
burrowing events and improve the anchorage of (Spix in Wagner, 1827) in the Piraju reservoir (São
animals that inhabit clayey sediments (Cândido Paulo state, southeastern Brazil). These values
& Romero 2006, 2007). In field and laboratory, it are significantly lower than those recorded in
was observed that large mussels were capable to the present study. This is possibly due to the
bury and escape faster than smaller animals (LRP difficulty of collecting these mussels, since
Paschoal personal data). Additionally, Callil et al. these animals live buried in deep substrates (up
(2018) have shown that fecundity in A. trapesialis to 15 m) and have large body sizes, which makes
is directly proportional to the size of the mussel, it difficult to obtain individuals by conventional
where large animals have higher offspring than collection devices such as nets and suction or
smaller individuals. In the analyzed reservoir, it grab samplers (SEMA 2013).
is likely that large animals had more chances to Several ecological factors influence the
escape the desiccation by burying and taking distribution of mollusks and among them
advantage of moist microhabitats, making these the substrate is a determinant factor in the
mussels more suitable for reproduction. distribution of freshwater bivalves (Harman 1972,
Anodontites trapesialis showed an Serrano et al. 1998, Paschoal et al. 2015). This
aggregation behavior with high density along characteristic was observed in the present study,
the stretch analyzed in the study area. The most since significant differences were observed for
common spatial distribution in bivalves is the the three quarters, in which only the first one
aggregate. This is due to the environmental differed from the others. The area occupied by
heterogeneity of the area, in which bivalves group Quarter I was composed of clayey substrate
in microenvironments more suitable for their and showed evidences of high concentration of
lifestyle (Santos et al. 2012, Paschoal et al. 2015). organic matter and abundance of Nile tilapia,
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 7 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
Table II. Total number of shells (N), mean, minimum and maximum density (Ind/m2), distribution pattern (IM)
and mean (± standard deviation) values of the analyzed biometric variables for the dead valves of Anodontites
trapesialis at the three quarters analyzed.
Quarter N Ind/m2 IM L H Wi
5.52a 111.25a 56.00a 35.40a
I 546 1.39
(1 - 22) (± 7.50) (± 4.05) (± 2.35)
1.05b 106.55b 53.45b 33.90b
II 104 1.64
(1 - 5) (± 10.75) (± 5.80) (± 3.40)
1.64b 109.45b 55.00b 34.80b
III 162 1.13
(1 - 6) (± 9.10) (± 4.95) (± 2.85)
Different letters represent significantly different means. H, shell height. L, shell length. Wi, shell width.
since this area was fully covered by grass and mortality in the A. trapesialis population, while
showed several nests of tilapia. An explanation juveniles were rarely observed. Bódis et al.
for this distribution pattern and higher density, (2014) when evaluating the massive mortality of
biomass and body proportions of A. trapesialis bivalves along the Danube River Basin (Hungary)
in the area of Quarter I would be given by the caused by drought, verified that the individuals
synergy of some factors: (I) the clayey substrate of the Chinese pond mussel Sinanodonta
(i.e. fine particles) of this area would aid the woodiana (Lea, 1834) with a size range of 60 ˫ 180
colonization and burrowing process of these mm L were the animals more affected. Semenas
mussels (Pereira et al. 2000, Cândido & Romero & Brugni (2002) suggest that the size distribution
2006, 2007), thus avoiding predation and of bivalves in limnic environments is modulated
desiccation, (II) due to the fact of the species by depth, and that smaller animals inhabit
is a suspension feeder that preferably inhabits deeper areas. Thus, it is possible that juveniles
regions of lower river flow with high amounts of A. trapesialis may not be affected as large
of phytoplankton (food resource) and organic adults when water levels decrease because they
matter (Simone 1994, Pereira et al. 2011), these inhabit deeper areas.
environmental features were observed in the In 2014, a new survey was carried out
studied area when not exposed to the drought to verify if the population presented or not
[see Paschoal et al. (2015) and Brito et al. (2016)], recovery in the number of individuals. However,
and (III) the presence and possible use of the it was not possible to be performed due to the
non-native fish O. niloticus as hosts for its larvae abundance of vegetation in the initial stage of
(Guardia-Felipi & Silva-Souza 2008), since we terrestrial succession at the sampled area (Fig.
detected several dead shells near to the tilapia S4), showing that the flooded area of Furnas
nests. reservoir did not recover from the drought (Fig.
Large adult individuals1 (100 ˫ 124 mm L) were 2). As verified by Bódis et al. (2014), the massive
more common during the analysis of massive mortality of bivalves promotes the input of
1
Here, we considered mature mussels all animals with sensu Callil & Mansur 2007). Mussels with small shells
> 47 mm L (size at the onset of physiological maturity, (< 47 mm L) were considered immature individuals.
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 8 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
Figure 5. Shell length-frequency
distributions of Anodontites
trapesialis dead shells (N: 812)
at Furnas HPS reservoir. Values
on the top of the columns are
corresponded to the total number
analyzed animals.
nutrients and biomass into the area where it after the flooding and reestablishment of the
occurs, increasing nutrient concentration and, normal water column levels.
consequently, primary productivity. In the The habitat modification promoted by
analyzed area, the concentration of organic dams (i.e. water level control) associated with
matter in sediment increased between two to climatic changes may restrict populations of A.
six times (128 g/kg) after the massive mortality. trapesialis and should be faced as an ecological
In addition, dead shells probably increased problem, since it is considered one of the
calcium concentration in the analyzed area causes of bivalve extinction (Bogan 1993, Haag &
(0.76 g/kg Ca), since nearby areas without A. Williams 2014). The present study shows the first
trapesialis had concentrations of this element, record of massive mortality of native bivalves
three times lower (~ 0.25 g/kg Ca) (L.R.P. Paschoal, in South America promoted by synergic events.
personal data). This shows that the shells can A management proposal for A. trapesialis and
persist in the environment, providing calcium other mussels, would be the relocation of these
and nutrients that will be incorporated into bivalves to nearby deeper areas with perennial
the sediment (Strayer & Malcom 2007). It can and resident water, aiming the conservation and
be noted that this phenomenon promoted an maintenance of these native species. We suggest
ecological impact in the analyzed area, altering monitoring of the populations and verification
the environmental scenario of the reservoir. of the effectiveness of the adopted measures,
Future studies should be carried out in this being the responsibility of the competent organs
area, in order to verify how nutrient and calcium the adoption of mitigating measures. In addition,
cycling occur from dead shells and how the it was observed in field the coexistence of the
ecological succession will impact the reservoir giant freshwater mussel to the Asian clam C.
fluminea (L.R.P. Paschoal, personal observation).
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 9 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
This invasive species has the capacity to alter and ST thank FCNyM (UNLP11/N927) and CONICET for the
the environment that inhabits and is capable to support. We are extremely thankful to the anonymous
reviewers that greatly improved the manuscript with
displace native species. Moreover, when exposed relevant suggestions.
to climatic disturbances it shows a fast recovery
and can overlap and repel native species in
the same area of occurrence (Sousa et al. 2008, REFERENCES
Paschoal et al. 2015). Until now, seven non-native
AGOSTINHO AA, JÚLIO JR HF & BORGHETTI JR . 1992.
species of mollusks were registered in reservoirs Considerações sobre os impactos dos represamentos
of Brazilian basins (Pereira et al. 2018). These na ictiofauna e medidas para sua atenuação. Um estudo
authors suggested that invasive species can de caso: Reservatório de Itaipu. Rev UNIMAR 14: 89-107.
promote a drastic reduction of native mollusk ANA - AGÊNCIA NACIONAL DE ÁGUAS. 2017. Sistema Nacional
populations, so an increased attention should de Informações sobre recursos hídricos. Brasil: ANA. URL:
<http://portalsnirh.ana.gov.br>. Acessado em 01.02.17.
be given to the monitoring of the A. trapesialis
ANDRADE DP, PASCHOAL LRP, RIGOLIN-SÁ O & FRANÇA N. 2012.
population that inhabits this reservoir.
Assessment of Hydroelectric Power Station Marechal
The development of conservation strategies Mascarenhas de Morais fifth-order tributaries water
for bivalves faces challenges, including the quality at the Rio Grande watershed (Minas Gerais State,
lack of quantitative data and/or assessments, Brazil). Acta Limnol Bras 24: 326-337.
selection of priority species and populations BAILLIE JEM, HILTON-TAYLOR C & STUART SN . 2004. 2004
for conservation, strategic decisions on habitat IUCN Red List of Threatened Species. A Global Species
Assessment. United Kingdom: IUCN, 191 p.
restoration and propagation in captivity, as well
as the participation of academic, governmental BÓDIS E, TÓTH B & SOUSA R. 2014. Massive mortality of
invasive bivalves as a potential resource subsidy for the
and general public (Régnier et al. 2009, Santos et
adjacent terrestrial food web. Hydrobiologia 735: 253-262.
al. 2013, Lopes-Lima et al. 2017, Torres et al. 2018).
BOGAN AE. 1993. Freshwater bivalve extinctions (Mollusca:
Thus, future studies related to temporal changes Unionoida): a search for causes. Am Zool 33: 599-609.
in the patterns of transitional environments are
BRASIL . 2012. MINISTÉRIO DO MEIO AMBIENTE. CONAMA -
of extreme importance, since the bivalves are CONSELHO NACIONAL DO MEIO AMBIENTE. Resolução nº 357,
also a faunal group with a high rate of extinction de 17 de março de 2005. Dispõe sobre a classificação
(Baillie et al. 2004, Lydeard et al. 2004), as well dos corpos de água e diretrizes ambientais para o seu
enquadramento, bem como estabelece as condições
as the mycetopodids (Pereira et al. 2012). The
e padrões de lançamento de efluentes, e dá outras
results presented here described the effects providências. Brasília: Ministério do Meio Ambiente, 1126
of extreme climatic events in Neotropical p.
reservoirs, creating a basis for future studies on BRITO SL, MAIA-BARBOSA PM & PINTO-COELHO RM . 2016.
ecology and conservation of freshwater native Secondary productivity of main microcrustacean species
bivalves. of two tropical reservoirs in Brazil and its relationship
with trophic state. J Limnol 75: 320-329.
Acknowledgments CALLIL CT, KRINSKI D & SILVA FA. 2012. Variations on the
We thank Dr. Luiz Simone (MZSP) for confirming species larval incubation of Anodontites trapesialis (Unionoida,
identification, Biologists Deivid P. Andrade and Thainá Mycetopodidae): Synergetic effect of the environmental
D. Franco for helping with field work, Dr. Norival França factors and host availability. Braz J Biol 72: 545-552.
for packing and shipment of the voucher samples to CALLIL CT, LEITE MC, MATEUS LA & JONES JW. 2018. Influence
MZSP, and Dr. Valter M. Azevedo-Santos for helping with of the flood pulse on reproduction and growth of
field work, identification of tilapia nests and for his Anodontites trapesialis (Lamarck, 1819) (Bivalvia:
comments which significantly improved this paper. GD
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 10 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
Mycetopodidae) in the Pantanal wetland, Brazil. HUXLEY JS. 1950. Relative growth and form transformation.
Hydrobiologia 810: 433-448. Proc R Soc Lond B Biol Sci 137: 465-469.
CALLIL CT & MANSUR MCD. 2007. Gametogênese e dinâmica ILARRI MI, ANTUNES C, GUILHERMINO L & SOUSA R . 2011.
da reprodução de Anodontites trapesialis (Lamarck) Massive mortality of the Asian clam Corbicula fluminea
(Unionoida: Mycetopodidae) no lago Baía do Poço, in a highly invaded area. Biol Invasions 13: 277-280.
planície de inundação do rio Cuiabá, Mato Grosso.
KREBS CJ. 1989. Ecological methodology. New York: Harper
Zoologia 24: 825-840.
& Row, 624 p.
CÂNDIDO LTS & ROMERO SMB. 2006. Heart rate and burrowing
LOPES-LIMA M ET AL . 2017. Conservation status of
behavior in the mussel Anodontites trapesialis (Bivalvia:
freshwater mussels in Europe: state of the art and future
Mycetopodidae) from lotic and lentic sites. Comp
challenges. Biol Rev 92: 572-607.
Biochem Physiol A Mol Integr Physiol 145: 131-136.
LYDEARD C ET AL. 2004. The global decline of nonmarine
CÂNDIDO LTS & ROMERO SMB. 2007. A contribution to the
mollusks. BioScience 54: 321-330.
knowledge of the behaviour of Anodontites trapesialis
(Bivalvia: Mycetopodidae). The effect of sediment type MELO DCD, SCANLON BR, ZHANG Z, WENDLAND E & YIN L. 2016.
on burrowing. Belg J Zool 137: 11-16. Reservoir storage and hydrologic responses to droughts
in the Paraná River basin, south-eastern Brazil. Hydrol
COLLAS FPL, KOOPMAN KR, HENDRIKS AJ, VAN DER VELDE G,
Earth Syst Sc 20: 4673-4688.
VERBRUGGE LNH & LEUVEN RSEW. 2014. Effects of desiccation
on native and non-native molluscs in rivers. Freshwater NASCIMENTO-FILHO SL, VIANA GFS & GOMES RLM . 2014.
Biol 59: 41-55. Inventário da malacofauna límnica de três grandes
reservatórios do sertão de Pernambuco, Brasil. Scientia
DARRIGRAN G & LAGRECA M. 2005. Moluscos Litorales del
Plena 10: 1-7.
Estuario del Río de la Plata. ProBiota. Buenos Aires: Serie
Técnica y Didactica nº 8, 41 p. NAZARENO AG & LAURANCE WF . 2015. Brazil’s drought:
Beware deforestation. Science 347: 1427.
DARRIGRAN G & LOPEZ-ARMENGOL MF. 1998. Composition,
structure and distribution of malacofauna living on a ONS - OPERADOR NACIONAL DO SISTEMA ELÉTRICO . 2017.
hard substrate at the Argentinian shore of Río de la Plata, Histórico da Operação. Brasil: ONS. URL: <http://http://
Argentina. Gayana 62: 79-89. www.ons.org. br>. Acessado em 01.02.17.
FAO - FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED PASCHOAL LRP, ANDRADE DP & DARRIGRAN G. 2015. How
NATIONS. 2016. AQUASTAT website. URL: <www.fao.org/nr/ the fluctuations of water levels affect populations of
water/aquastat/dams/index.stm>. Accessed: 01.02.17. invasive bivalve Corbicula fluminea (Müller, 1774) in a
Neotropical reservoir? Braz J Biol 75: 135-143.
GRAF DL & CUMMINGS KV. 2007. Review of the systematics
and global diversity of freshwater mussel species (Bivalvia: PASCHOAL LRP, STRIPARI NL & CARVALHO K. 2012. Estrutura
Unionoida). J Molluscan Stud 73: 291-314. da comunidade de macroinvertebrados bentônicos
no reservatório da usina hidréletrica de Furnas, Minas
GUARDIA-FELIPI P & SILVA-SOUZA AT. 2008. Anodontites
Gerais, Brasil. In: Rigolin-Sá O (Ed), Bacia Hidrográfica:
trapesialis (Lamarck, 1819): um bivalve parasito de peixes
Estudos do Rio Grande no Sudoeste de Minas Gerais -
de água doce. Semina: Ciências Agrárias 29: 895-904.
Brasil. Edifesp: Passos, p. 169-182.
HAAG WR & WILLIAMS JD. 2014. Biodiversity on the brink: an
PEREIRA D, ARRUDA JO, MENEGAT R, PORTO ML, SCHWARZBOLD
assessment of conservation strategies for North American
A & HARTZ SM . 2011. Guildas tróficas, composição e
freshwater mussels. Hydrobiologia 735: 45-60.
distribuição de espécies de moluscos límnicos no
HARMAN WN. 1972. Benthic substrates: their effect on gradiente fluvial de um riacho subtropical brasileiro.
freshwater Mollusca. Ecology 53: 271-277. Biotemas 24: 21-36.
HENRY R. 1999. Ecologia de reservatórios: estrutura, função PEREIRA D ET AL. 2014. Bivalve distribution in hydrographic
e aspectos sociais. Botucatu: Fapesp/Fundibio Brazil, 800 regions in South America: historical overview and
p. conservation. Hydrobiologia 735: 15-44.
HENRY R & SIMÃO CA . 1986. Abundância, diversidade PEREIRA D, MANSUR MCD & PIMPÃO DM. 2012. Identificação
e biomassa de Mollusca na represa de Piraju (rio e diferenciação dos bivalves límnicos invasores dos
Paranapanema, SP). Rev Bras Biol 46: 507-516. demais bivalves nativos do Brasil. In: Mansur MCD,
Santos CP, Pereira D, Paz ICP, Zurita MLL, Rodriguez MTR,
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 11 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
Nehrke MV and Bergonci PEA (Eds), Moluscos límnicos respect to the terrestrial and freshwater ecosystems.
invasores no Brasil: biologia, prevenção e controle. São Paulo: FAPESP, 390 p.
Redes Editora: Porto Alegre, p. 75-94.
SOUSA R, ANTUNES C & GUILHERMINO L. 2008. Ecology of the
PEREIRA D, VEITENHEIMER-MENDES IL, MANSUR MCD & SILVA invasive Asian clam Corbicula fluminea (Müller, 1774) in
MCP. 2000. Malacofauna límnica no sistema de irrigação aquatic ecosystems: an overview. Ann Limnol Int J Lim
da microbacia do arroio Capivara, Triunfo, RS, Brasil. 44: 85-94.
Biociências 8: 137-157.
SOUSA R, FERREIRA A, CARVALHO F, LOPES-LIMA M, VARANDAS S &
PEREIRA LS, NEVES RDAF, MIYAHIRA IC, KOZLOWSKY-SUZUKI TEIXEIRA A. 2018. Die-offs of the endangered pearl mussel
B, BRANCO CWC, PAULA JC & SANTOS LN. 2018. Non-native Margaritifera margaritifera during an extreme drought.
species in reservoirs: how are we doing in Brazil? Aquatic Conserv Mar Freshw Ecosyst 28: 1244-1248.
Hydrobiologia 817: 71-84.
SOUSA R, VARANDAS S, CORTES R, TEIXEIRA A, LOPES-LIMA M,
PILGER TJ & GIDO KB. 2012. Variation in Unionid assemblages MACHADO J & GUILHERMINO L. 2012. Massive die-offs of
between streams and a reservoir within the Kansas River freshwater bivalves as resource pulses. Ann Limnol Int
Basin. Am Midl Nat 167: 356-365. J Lim 48: 105-112.
RÉGNIER C, FONTAINE B & BOUCHET P. 2009. Not knowing, STRAYER DL & MALCOM HM. 2007. Shell decay rates of native
not recording, not listing: numerous unnoticed mollusk and alien freshwater bivalves and implications for
extinctions. Conserv Biol 23: 1214-1221. habitat engineering. Freshwater Biol 52: 1611-1617.
SANTOS SB, MIYAHIRA IC & MANSUR MCD. 2013. Freshwater TORRES S, CAO L, GUTIERREZ GREGORIC D, DE LUCIA M, BREA F &
and terrestrial molluscs in Brasil: current status of DARRIGRAN G. 2018. Distribution of the Unionida (Bivalvia,
knowledge and conservation. Tentacle 21: 40-42. Paleoheterodonta) from Argentina and its conservation
in the Southern Neotropical Region. PLoS ONE 13: 1-15.
SANTOS SB, THIENGO SC, FERNANDEZ MA, MIYAHIRA IC,
GONÇALVES IB, XIMENES RDF, MANSUR MCD & PEREIRA D. 2012. VAUGHN CC, ATKINSON CL & JULIAN JP. 2015. Drought-induced
Espécies de moluscos límnicos invasores no Brasil. changes in flow regimes lead to long-term losses
In: Mansur MCD, Santos CP, Pereira D, Paz ICP, Zurita in mussel-provided ecosystem services. Ecol Evol 5:
MLL, Rodriguez MTR, Nehrke MV and Bergonci PEA 1291-1305.
(Eds), Moluscos límnicos invasores no Brasil: biologia,
prevenção e controle. Redes Editora: Porto Alegre, p.
25-50.
SUPPLEMENTARY MATERIAL
SEMA - SECRETÁRIA ESTADUAL DO MEIO AMBIENTE. 2013. Ficha
de Informações para Avaliação da espécie. Anodontites Figure S1.
trapesialis Lamarck, 1819. In: Oficina de avaliação do Figure S2.
estado de conservação dos Invertebrados Marinhos da Figure S3.
Bahia. Secretária Estadual do Meio Ambiente: Ilhéus, p. Figure S4.
01.
SEMENAS L & BRUGNI N. 2002. Características poblacionales How to cite
y ciclo de vida de Diplodon chilensis (d’Orbigny, 1835) PASCHOAL LRP, ANDRADE DP, PIMPÃO DM, TORRES S & DARRIGRAN G.
(Hyriidae, Bivalvia) en el lago Gutiérrez (Patagonia, 2020. Massive mortality of the giant freshwater mussel Anodontites
trapesialis (Lamarck, 1819) (Bivalvia: Mycetopodidae) during a severe
Argentina). Ecol Austral 12: 29-40.
drought in a Neotropical reservoir. An Acad Bras Cienc 92: e20180811.
SERRANO MAS, TIETBÖHL RS & MANSUR MCD. 1998. Sobre a DOI 10.1590/0001-3765202020180811.
ocorrência de moluscos bivalves no Pantanal de Mato
Grosso, Brasil. Biociências 6: 131-144.
Manuscript received on August 7, 2018;
SIMONE LRL. 1994. Anatomical characters and systematics accepted for publication on May 7, 2019
of Anodontites trapesialis (Lamarck, 1819) from South
America (Mollusca, Bivalvia, Unionoida, Muteloidea). LUCAS R.P. PASCHOAL1
Stud Neotrop Fauna E 29: 169-185. https://orcid.org/0000-0003-2461-4675
SIMONE LRL. 2006. Land and freshwater molluscs of Brazil:
DOUGLAS P. ANDRADE2
an illustrated inventory on the Brazilian malacofauna, https://orcid.org/0000-0002-5354-5309
including neighbour regions of the South America,
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 12 | 13
LUCAS R.P. PASCHOAL et al. MASSIVE DIE-OFF OF Anodontites trapesialis
DANIEL M. PIMPÃO3
https://orcid.org/0000-0002-3958-5099
SANTIAGO TORRES4
https://orcid.org/0000-0002-2118-0739
GUSTAVO DARRIGRAN5
https://orcid.org/0000-0001-9512-8135
1
Faculdade de Tecnologia Nilo de Stéfani/FATEC, Av. Eduardo
Zambianchi, 31, V. Industrial, 14883-130 Jaboticabal, SP, Brazil
2
Universidade Federal de São Carlos/UFSCar, Rod. Washington
Luís, Km 235, SP-310, 13565-905 São Carlos, SP, Brazil
3
IBAMA - Instituto Brasileiro do Meio Ambiente e dos
Recursos Naturais Renováveis, Superintendência
Regional, Rua Conselheiro Mafra, 784, Centro,
88010-102 Florianópolis, SC, Brazil
4
Centro de Investigaciones y Transferencia Santa Cruz
(CONICET, UNPA, UTN), Unidad Académica San Julián,
Universidad Nacional de la Patagonia Austral, Cristóbal Colón,
1570, Puerto San Julian Santa Cruz, Santa Cruz, Argentina
5
Museo de La Plata (FCNyM-UNLP), División
Zoologia Invertebrados, CONICET,
Paseo del Bosque, s/n°, La Plata, 1900, Argentina
Correspondence to: Lucas Rezende Penido Paschoal
E-mail: lucasrezende20@gmail.com
Author contributions
Each author have contributed significantly to the intellectual
content of the work, specifically: Dr. LRPP - Conceptualization,
Data sampling, analysis and interpretation, Writing original
draft preparation, Writing-Reviewing and Editing, Dr. DPA - Data
sampling, Writing original draft preparation, Writing-Reviewing
and Editing, Dr. DMP and Dr. GD - Conceptualization, Writing
original draft preparation, Supervision and Writing-Reviewing
last version, Dr. ST - Conceptualization, Writing original draft
preparation and Writing-Reviewing last version.
An Acad Bras Cienc (2020) 92(Suppl. 2) e20180811 13 | 13
You might also like
- Build A Borz Practical Scrap Metal Small Arms Vol9Document23 pagesBuild A Borz Practical Scrap Metal Small Arms Vol9Gia Linh Văn100% (2)
- Modal Analysis of Beams - An Experiment Symposium On Dynamic Problems of MechanicsDocument9 pagesModal Analysis of Beams - An Experiment Symposium On Dynamic Problems of MechanicsnizamshahrinNo ratings yet
- Mathematics 2nd Quarter Test With TOSDocument8 pagesMathematics 2nd Quarter Test With TOSRona Mae Aira AvilesNo ratings yet
- Mixed Use DevelopmentDocument3 pagesMixed Use DevelopmentMark Darcy UngsonNo ratings yet
- L1 Finding Nemo Teacher Notes American EnglishDocument9 pagesL1 Finding Nemo Teacher Notes American Englishcris_simescuNo ratings yet
- IV Solution Cheat Sheet: Type Description Osmolality Use MiscellaneousDocument1 pageIV Solution Cheat Sheet: Type Description Osmolality Use MiscellaneousKristine Castillo100% (2)
- Modelling beta diversity of macroinvertebrates in Andean wetlandsDocument16 pagesModelling beta diversity of macroinvertebrates in Andean wetlandsJeffrey PrietoNo ratings yet
- Patrones Espaciales en Comunidades de Macroinvertebrados Acuáticos de La Puna ArgentinaDocument16 pagesPatrones Espaciales en Comunidades de Macroinvertebrados Acuáticos de La Puna Argentinadanielvargas1No ratings yet
- Quaternary Science ReviewsDocument12 pagesQuaternary Science ReviewsKarely BalcazarNo ratings yet
- Arvalofras2014 EmbryoDocument14 pagesArvalofras2014 Embryoanon_509992240No ratings yet
- Ibarra-Vidal Et Al. 2022 Salinidad P Taul y B TaeniataDocument5 pagesIbarra-Vidal Et Al. 2022 Salinidad P Taul y B TaeniataHéctor Ibarra VidalNo ratings yet
- Benthic Community Recovery From Brine Impact After The Implementation of Mitigation MeasuresDocument12 pagesBenthic Community Recovery From Brine Impact After The Implementation of Mitigation MeasuresBrigita de BrillarNo ratings yet
- Ecology and Distribution of Aulacoseira Species Bacillariophyta in Tropical Reservoirs From BrazilDocument18 pagesEcology and Distribution of Aulacoseira Species Bacillariophyta in Tropical Reservoirs From BrazilPaola JiradoNo ratings yet
- Represa de PradoDocument12 pagesRepresa de PradoMarcela CamachoNo ratings yet
- 1 s2.0 S0025326X22009900 MainDocument10 pages1 s2.0 S0025326X22009900 MainWilbert PerezNo ratings yet
- Bioacumulation of Trace Elements in The Crab Ucides Cordatus PDFDocument15 pagesBioacumulation of Trace Elements in The Crab Ucides Cordatus PDFBruna Mariáh FernandesNo ratings yet
- Phytoplankton Functional Groups in a Brazilian ReservoirDocument13 pagesPhytoplankton Functional Groups in a Brazilian ReservoirfrfunkNo ratings yet
- Porsopis TamarugoPhil A Native Tree From The Atacama Desert Groundwater Table Depth Thresholds For ConservationDocument8 pagesPorsopis TamarugoPhil A Native Tree From The Atacama Desert Groundwater Table Depth Thresholds For ConservationJavier Carrasco SologurenNo ratings yet
- Hydroclimatology of The Upper Madeira River Basin Spatio Temporal Variability and TrendsDocument18 pagesHydroclimatology of The Upper Madeira River Basin Spatio Temporal Variability and TrendsDiegoNo ratings yet
- Ecology and Evolution - 2021 - Lopera Congote - River Connectivity and Climate Behind The Long Term Evolution of TropicalDocument19 pagesEcology and Evolution - 2021 - Lopera Congote - River Connectivity and Climate Behind The Long Term Evolution of TropicalPaola JiradoNo ratings yet
- 2023-Gurgel-Lourenco - Checklis Fish Fauna The Estuaries of CearaDocument28 pages2023-Gurgel-Lourenco - Checklis Fish Fauna The Estuaries of CearaFrancisco ClebsonNo ratings yet
- Scientific Note Size Comparison of Quadrats in Sample of Non-Native Bivalve CorbiculaDocument6 pagesScientific Note Size Comparison of Quadrats in Sample of Non-Native Bivalve CorbiculaLucas PaschoalNo ratings yet
- 2008 CorraUieda Faunamangue PanamJASDocument10 pages2008 CorraUieda Faunamangue PanamJASJhon Patrick DelmonteNo ratings yet
- Paschoal Et Al. (2015) - Brazilian Journal of Biology - 75 (1) - 135-143Document9 pagesPaschoal Et Al. (2015) - Brazilian Journal of Biology - 75 (1) - 135-143Lucas PaschoalNo ratings yet
- Freshwater decapod crustaceans of southeast BahiaDocument30 pagesFreshwater decapod crustaceans of southeast BahiaAndré Ramos CostaNo ratings yet
- Ubirajara Et Al 2017 Impact of Filamentous Cyanobacteria On The Water Quality of Two Tropical ReservoirsDocument11 pagesUbirajara Et Al 2017 Impact of Filamentous Cyanobacteria On The Water Quality of Two Tropical ReservoirsfrfunkNo ratings yet
- Diversidad, Hábito y Hábitat de Macrófitos PDFDocument20 pagesDiversidad, Hábito y Hábitat de Macrófitos PDFLuis GarciaNo ratings yet
- Climate Changes and Human Activities RecDocument30 pagesClimate Changes and Human Activities RecHalsandNo ratings yet
- 0034 7744 RBT 71 01 E52779Document17 pages0034 7744 RBT 71 01 E52779Luisa SGNo ratings yet
- Mesozooplankton grazing in eutrophic Guanabara BayDocument10 pagesMesozooplankton grazing in eutrophic Guanabara BayMilton Luiz Vieira AraujoNo ratings yet
- A Comparison of The Wood Anatomy of 11 Species From Two Cerrado Habitats (Cerrado S.S. and Adjacent Gallery Forest) - Sonsin Et AlDocument20 pagesA Comparison of The Wood Anatomy of 11 Species From Two Cerrado Habitats (Cerrado S.S. and Adjacent Gallery Forest) - Sonsin Et Allihelih1No ratings yet
- Barberis Et Al 2023 Tank Bromeliads As A Water Reservoir Used by Humans An Important Overlooked Ecosystem Service inDocument13 pagesBarberis Et Al 2023 Tank Bromeliads As A Water Reservoir Used by Humans An Important Overlooked Ecosystem Service inflopodestaNo ratings yet
- 1 s2.0 S0048969719302323 MainDocument14 pages1 s2.0 S0048969719302323 MainMas'ud KholahNo ratings yet
- Fishes From The Las Piedras River, Madre de Dios Basin, Peruvian Amazon - Carvalho Et Al. - 2012Document47 pagesFishes From The Las Piedras River, Madre de Dios Basin, Peruvian Amazon - Carvalho Et Al. - 2012Dayvis RH MontesNo ratings yet
- Lopez Et Al, 2015 PDFDocument14 pagesLopez Et Al, 2015 PDFMario HerreraNo ratings yet
- Vegetacinacuticadeloshumedalesdelaquebrada EsteroDocument13 pagesVegetacinacuticadeloshumedalesdelaquebrada EsteroEmmanuel MejiasNo ratings yet
- Arias Et Al. - 2021 - Hydroclimate of The Andes Part II Hydroclimate Va-AnnotatedDocument25 pagesArias Et Al. - 2021 - Hydroclimate of The Andes Part II Hydroclimate Va-AnnotatedAlfredo Dex Quispe MarrónNo ratings yet
- Medium and Large Sized Mammals of The Boqueirão Da Onça, North of Bahia State, BrazilDocument8 pagesMedium and Large Sized Mammals of The Boqueirão Da Onça, North of Bahia State, BrazilAdilson Rodrigues RosaNo ratings yet
- 1 s2.0 S0048969722050070 MainDocument14 pages1 s2.0 S0048969722050070 MainVerónika R MRNo ratings yet
- MacroninvertebradosDocument20 pagesMacroninvertebradosdanielvargas1No ratings yet
- Angeli Et Al. 2019. Environmental Changes Reflected by Sedimentary Geochemistry For The Las One Hundred Years of A Tropical EstuaryDocument14 pagesAngeli Et Al. 2019. Environmental Changes Reflected by Sedimentary Geochemistry For The Las One Hundred Years of A Tropical EstuaryJosé Lourenço AngeliNo ratings yet
- Rocha 2004Document17 pagesRocha 2004Jaranna CoelhoNo ratings yet
- Articulo 15Document18 pagesArticulo 15CAMILO LINDARTENo ratings yet
- Food Web Structure in The Xingu River Rapids PriorDocument19 pagesFood Web Structure in The Xingu River Rapids PriorEsther Mirian Cardoso MesquitaNo ratings yet
- Taufik AmbioDocument13 pagesTaufik Ambiomobster44No ratings yet
- Biogardens As Constructed Wetlands in Tropical Climate Costa RicaDocument6 pagesBiogardens As Constructed Wetlands in Tropical Climate Costa RicaLaura HernandezNo ratings yet
- Marine Pollution BulletinDocument7 pagesMarine Pollution BulletinPhuping SucharitakulNo ratings yet
- Structure and diversity of three plant associations in the San Juan River DeltaDocument11 pagesStructure and diversity of three plant associations in the San Juan River DeltaCamil O'Hara MilloNo ratings yet
- Lavado Casimiro Et Al. - 2011 - Assessment of Climate Change Impacts On The Hydrology of The Peruvian Amazon-Andes Basin-AnnotatedDocument14 pagesLavado Casimiro Et Al. - 2011 - Assessment of Climate Change Impacts On The Hydrology of The Peruvian Amazon-Andes Basin-AnnotatedAlfredo Dex Quispe MarrónNo ratings yet
- 13-MENDEZ-Condición Del Arrecife Coralino de Playa BlancaDocument14 pages13-MENDEZ-Condición Del Arrecife Coralino de Playa BlancammendezbioNo ratings yet
- Fpls 09 00196Document14 pagesFpls 09 00196luengunaNo ratings yet
- 2020 Article 1316Document17 pages2020 Article 1316Hermes De GraciaNo ratings yet
- Gonzalez Et Al 10 EMASDocument13 pagesGonzalez Et Al 10 EMASKevin Fernando Salazar CoquincheNo ratings yet
- Yacobaccio, Hugo Et Al. (2016) - Habitats of Ancient Hunter-Gatherers in The Puna. Resilience and Discontinuities During The HoloceneDocument9 pagesYacobaccio, Hugo Et Al. (2016) - Habitats of Ancient Hunter-Gatherers in The Puna. Resilience and Discontinuities During The HoloceneTreder Jauregui CassiaNo ratings yet
- Species Inventory of Aquatic Macrophytes in The LaDocument15 pagesSpecies Inventory of Aquatic Macrophytes in The LaKim LimboNo ratings yet
- 2015 María Mujica - Late Quaternary Climate Change, Relict PopulationsDocument13 pages2015 María Mujica - Late Quaternary Climate Change, Relict PopulationsCarlos UrizarNo ratings yet
- Surface-Watergroundwater Exchange in A Sand Dune Lake in The Dry aRGENTINAiSOTOPOSDocument14 pagesSurface-Watergroundwater Exchange in A Sand Dune Lake in The Dry aRGENTINAiSOTOPOSCASTAÑEDA GANTIVA JAIME ANDRÉSNo ratings yet
- Asociaciones PecesDocument13 pagesAsociaciones PecesjorgelopezrochaNo ratings yet
- Risks of Dam Construction For South American River Dolphins: A Case Study of The Tapajós RiverDocument14 pagesRisks of Dam Construction For South American River Dolphins: A Case Study of The Tapajós RiverMiriam MarmontelNo ratings yet
- Plant Species Diversity in A NeotropicalDocument13 pagesPlant Species Diversity in A Neotropicallucía espitiaNo ratings yet
- Castillo2018 PDFDocument13 pagesCastillo2018 PDFjoancoasNo ratings yet
- 1 PBDocument7 pages1 PBMylena NecoNo ratings yet
- IssnDocument13 pagesIssn3333No ratings yet
- 173-Inorganic Composition of Suspended Sediments in The Acre River, Amazon Basin, Brazil-2014Document14 pages173-Inorganic Composition of Suspended Sediments in The Acre River, Amazon Basin, Brazil-2014vere blancoNo ratings yet
- Ecohydrology & Hydrobiology: Giovany Guevara, Roberto Godoy, Pascal Boeckx, Carlos Jara, Carlos Oyarzu NDocument11 pagesEcohydrology & Hydrobiology: Giovany Guevara, Roberto Godoy, Pascal Boeckx, Carlos Jara, Carlos Oyarzu NIsabel M. RojasNo ratings yet
- Floodplain Wetland Biota in the Murray-Darling Basin: Water and Habitat RequirementsFrom EverandFloodplain Wetland Biota in the Murray-Darling Basin: Water and Habitat RequirementsNo ratings yet
- Protection in The Nuclear Age - FEMA H-20Document42 pagesProtection in The Nuclear Age - FEMA H-20tsakonasfotiosNo ratings yet
- 1998 - Camp Et Al. - Perkins Checklists of Selected Shallow-Water Marine Invertebrates of FloridaDocument256 pages1998 - Camp Et Al. - Perkins Checklists of Selected Shallow-Water Marine Invertebrates of FloridaPedro HernandezNo ratings yet
- Paschoal & Zara (2020) - AABCDocument19 pagesPaschoal & Zara (2020) - AABCLucas PaschoalNo ratings yet
- Paschoal Et Al. (2020) - AABC (SM)Document2 pagesPaschoal Et Al. (2020) - AABC (SM)Lucas PaschoalNo ratings yet
- Paschoal & Zara (2020) - AABC (SM)Document5 pagesPaschoal & Zara (2020) - AABC (SM)Lucas PaschoalNo ratings yet
- Paschoal Et Al. (2015) - Brazilian Journal of Biology - 75 (1) - 135-143Document9 pagesPaschoal Et Al. (2015) - Brazilian Journal of Biology - 75 (1) - 135-143Lucas PaschoalNo ratings yet
- Scientific Note Size Comparison of Quadrats in Sample of Non-Native Bivalve CorbiculaDocument6 pagesScientific Note Size Comparison of Quadrats in Sample of Non-Native Bivalve CorbiculaLucas PaschoalNo ratings yet
- Paschoal Et Al. (2018) - PANAMJAS - 13 (4) - 306-310 PDFDocument5 pagesPaschoal Et Al. (2018) - PANAMJAS - 13 (4) - 306-310 PDFLucas PaschoalNo ratings yet
- Paschoal Et Al. (2013) - Iheringia, Serie Zoológica - 103 (1) - 31-36 PDFDocument6 pagesPaschoal Et Al. (2013) - Iheringia, Serie Zoológica - 103 (1) - 31-36 PDFLucas PaschoalNo ratings yet
- Andrade Et Al. (2012) - Acta Limnologica Brasiliensia - 24 (3) - 326-337Document12 pagesAndrade Et Al. (2012) - Acta Limnologica Brasiliensia - 24 (3) - 326-337Lucas PaschoalNo ratings yet
- Role of Macrophytes in Distribution of Phytophilous Shrimp SpeciesDocument4 pagesRole of Macrophytes in Distribution of Phytophilous Shrimp SpeciesLucas PaschoalNo ratings yet
- PPG Hi-Temp™ 1027 HDDocument5 pagesPPG Hi-Temp™ 1027 HDMarleo MorenoNo ratings yet
- Sui Gas Connection FormsDocument1 pageSui Gas Connection Formsare50% (2)
- Chip Mong Noro Mall: Property OverviewDocument4 pagesChip Mong Noro Mall: Property OverviewHe VansakNo ratings yet
- Taylor Ebooks 4 Web 0218Document360 pagesTaylor Ebooks 4 Web 0218aquahellNo ratings yet
- A Perspective in Accelerated Orthodontics With Aligner Treatment 2017 Seminars in OrthodonticsDocument7 pagesA Perspective in Accelerated Orthodontics With Aligner Treatment 2017 Seminars in Orthodonticsdruzair007No ratings yet
- Review of oxidative stress and antioxidants in dentistryDocument4 pagesReview of oxidative stress and antioxidants in dentistrySeeptianMaulanaNo ratings yet
- Declaration Letter - Vetagro To GSDocument3 pagesDeclaration Letter - Vetagro To GSVíctor RodríguezNo ratings yet
- On K-Distance Degree Index of TreesDocument5 pagesOn K-Distance Degree Index of TreesVelumani sNo ratings yet
- Slide No. 04 - Pert - Cpm1Document27 pagesSlide No. 04 - Pert - Cpm1NUSRAT RITUNo ratings yet
- CP 108: Home Assignment Topics-2021Document2 pagesCP 108: Home Assignment Topics-2021Biswajit PaulNo ratings yet
- ReviewerDocument2 pagesReviewerFrank Jomari MurilloNo ratings yet
- Sanitary Layout Plan: Third FloorDocument1 pageSanitary Layout Plan: Third FloorFrancis KoNo ratings yet
- Chapter 11: The EyesDocument26 pagesChapter 11: The Eyesriley2021No ratings yet
- SP 5300DN SP 5310DN SÑljguideDocument32 pagesSP 5300DN SP 5310DN SÑljguidepaypkerry_179706015No ratings yet
- Zaldivar Et Al. 2017 (Dopamine Is Signaled by Mid-Frequency Oscillations and Boosts Output Layers Visual Information in Visual Cortex)Document30 pagesZaldivar Et Al. 2017 (Dopamine Is Signaled by Mid-Frequency Oscillations and Boosts Output Layers Visual Information in Visual Cortex)FRANCISCO ELI LEZAMA GUTIERREZNo ratings yet
- Kant ParadigmDocument265 pagesKant ParadigmkairospandemosNo ratings yet
- The Volcanic Explosivity Index (VEI)Document8 pagesThe Volcanic Explosivity Index (VEI)Rakhmatul ArafatNo ratings yet
- Abstract of SURYA NAMASKARDocument23 pagesAbstract of SURYA NAMASKARSarita SharmaNo ratings yet
- Brazed Tool ArDocument5 pagesBrazed Tool ArRoni MustafiqNo ratings yet
- Intel Processors PDFDocument33 pagesIntel Processors PDFbiplab royNo ratings yet
- Study Journal Lesson 23-32 - LisondraDocument3 pagesStudy Journal Lesson 23-32 - Lisondrasenior highNo ratings yet
- OK - EndUserGuideHoseSafetyInstituteDocument8 pagesOK - EndUserGuideHoseSafetyInstituteSunil GhosalkarNo ratings yet
- WB City GateDocument7 pagesWB City GateDiegoNo ratings yet
- 016 Muscoril COPPDocument3 pages016 Muscoril COPPTheRoom23No ratings yet