Professional Documents
Culture Documents
Thermodynamics PDF
Uploaded by
Ammar RizwanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermodynamics PDF
Uploaded by
Ammar RizwanCopyright:
Available Formats
Chemical Energetics F322 1
THERMODYNAMICS
First Law Energy can be neither created nor destroyed but It can be converted from one
form to another.
• all chemical reactions are accompanied by some form of energy change
• changes can be very obvious (gas burning) but in many cases it goes unnoticed
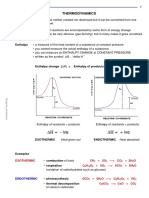
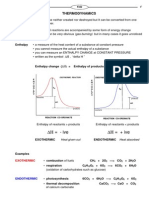
Enthalpy • a measure of the heat content of a substance at constant pressure
• you cannot measure the actual enthalpy of a substance
• you can measure an ENTHALPY CHANGE at CONSTANT PRESSURE
• written as the symbol ∆Η , “delta H ”
Enthalpy change (∆Η) = Enthalpy of products - Enthalpy of reactants
EXOTHERMIC REACTION ENDOTHERMIC REACTION
E E
N
N
T
T
H H
A Ea
A Ea
L
P L
P
Y
Y
reactants
products
H
H
products
reactants
Enthalpy of reactants > products Enthalpy of reactants < products
∆Η = - ive ∆Η = + ive
EXOTHERMIC Heat given out ENDOTHERMIC Heat absorbed
Examples
EXOTHERMIC • combustion of fuels CH4 + 2O2 —> CO2 + 2H2O
• respiration C6H12O6 + 6O2 —> 6CO2 + 6H2O
(oxidation of carbohydrates such as glucose)
ENDOTHERMIC • photosynthesis 6CO2 + 6H2O —> C6H12O6 + 6O2
• thermal decomposition CaCO3 —> CaO + CO2
of calcium carbonate
© KNOCKHARDY PUBLISHING 2009
2 F322 Chemical Energetics
Standard Enthalpy Changes
• enthalpy values vary with the conditions - so standard conditions are needed
• a substance will then be in its standard state ...
Pressure:- 100 kPa (1 atm) A stated temperature:- usually 298K (25°C)
• as a guide, just think of a substance under normal laboratory conditions
• assign the correct subscript [ e.g. (g), (l) or (s) ] to indicate which state it is in
• any solutions are of concentration 1 mol dm-3
• to tell if standard conditions are used we modify the symbol for ∆Η .
Enthalpy Change Standard Enthalpy Change (at 298K)
∆Η ∆Η 298
Standard Enthalpy Change of Combustion ( ∆Η°c )
Definition The enthalpy change when ONE MOLE of a substance undergoes complete
combustion under standard conditions. All reactants and products are in their
standard states.
Values Always exothermic
Example(s) C(graphite) + O2(g) ——> CO2(g)
C2H5OH(l) + 3O2(g) ——> 2CO2(g) + 3H2O(l)
Notes To aid balancing the equation, remember that you get one carbon dioxide
molecule for every carbon atom in the original molecule and a water molecule for
every two hydrogen atoms. Having done this, go back and balance the oxygen.
Q.1 Write equations representing the standard enthalpy change of combustion of...
methane
methanol
cyclohexane
hydrogen
carbon
© KNOCKHARDY PUBLISHING 2009
Chemical Energetics F322 3
Standard Enthalpy Change of Formation ( ∆Η°f )
Definition The enthalpy change when ONE MOLE of a compound is formed in its standard
state from its elements in their standard states.
Values Usually, but not exclusively, exothermic
Example(s) 2C(graphite) + ½O2(g) + 3H2(g) ——> C2H5OH(l)
Notes • Elements In their standard states have zero enthalpy of formation.
• Carbon is usually taken as the graphite allotrope.
Q.2 Construct equations representing the standard enthalpy change of formation of
methane
sulphuric acid
sodium chloride
water
carbon dioxide
Q.3 What do you notice about the equations for..
• the standard enthalpy change of combustion of hydrogen and the standard
enthalpy change of formation of water?
• the standard enthalpy change of combustion of carbon and the standard
enthalpy change of formation of carbon dioxide?
Enthalpy of Neutralisation
Definition Enthalpy change when ONE MOLE of water is formed from its ions in dilute soln.
Values Exothermic
Equation H+(aq) + OH¯(aq) ——> H2O(l)
Notes A value of -57kJ mol-1 is obtained when strong acids react with strong alkalis.
© KNOCKHARDY PUBLISHING 2009
4 F322 Chemical Energetics
Bond Dissociation Enthalpy (Energy)
Definition Energy required to break ONE MOLE of gaseous bonds to form gaseous atoms.
Values Endothermic Energy must be put in to break any chemical bond
Example Cl2(g) ——> 2Cl(g)
Notes • the strength of a bond depends on its environment so MEAN values are quoted
• making a bond is an exothermic process as it is the opposite of breaking a bond
• for diatomic gases, the bond enthalpy is twice the enthalpy of atomisation
• the smaller the bond enthalpy, the weaker the bond and the easier it is to break
Some mean bond enthalpies (in kJ mol-1)
(values may differ slightly in other texts)
H-H 436 H-F 562 N-N 163
C-C 346 H-Cl 431 N=N 409
C=C 611 H-Br 366 N≡N 944
C≡C 837 H-I 299 P-P 172
C-O 360 H-N 388 F-F 158
C=O 743 H-O 463 Cl-Cl 242
C-H 413 H-S 338 Br-Br 193
C-N 305 H-Si 318 I-I 151
C-F 484 P-H 322 S-S 264
C-Cl 338 O-O 146 Si-S 176
C-Br 276 O=O 496 Si-O 374
HESS’S LAW “The enthalpy change is independent of the path taken”
∆Ηr
A B
∆Η1 ∆Η3
∆Η2
X Y
∆Ηr = ∆Η1 + ∆Η2 + ∆Η3
• applying Hess’s Law enables one to calculate enthalpy changes from other data
• used for calculating changes which can’t be measured directly - Lattice Enthalpy
• used for calculating - enthalpy change of reaction from bond enthalpy
- enthalpy change of reaction from ∆Η°c
- enthalpy change of formation from ∆Η°f
© KNOCKHARDY PUBLISHING 2009
Chemical Energetics F322 5
Enthalpy change of reaction from average bond enthalpies
Theory Imagine that, during a reaction, all the bonds of reacting species are broken and
the individual atoms join up again but in the form of products. The overall energy
change will depend on the difference between the energy required to break the
bonds and that released as bonds are made.
energy released making bonds > energy used to break bonds ... EXOTHERMIC
energy used to break bonds > energy released making bonds ... ENDOTHERMIC
Example Calculate the enthalpy change for the hydrogenation of ethene
H H
∆Η1 H H
C C + H H H C C H
H H
H H
Breaking bonds - ∆Η2 ∆Η3 Breaking bonds -
ENDOTHERMIC ENDOTHERMIC
H H H
H C C H
H
∆Η2 1 x C = C bond @ 611 = 611
4 x C H bonds @ 413 = 1652
1 x H H bond @ 436 = 436
Total energy required to BREAK bonds of reactants = 2699 kJ mol -1
∆Η3 1 x C
6 x C
C bond @ 346 = 346
H bonds @ 413 = 2478
-1
Total energy required to BREAK bonds of products = 2824 kJ mol
Applying HESS'S LAW ∆Η1 = ∆Η2 - ∆Η3
= 2699 - 2824 = -125kJ
Q.4 Using the average bond enthalpies in your notes, calculate the
standard enthalpy changes of reaction for the following reactions.
a) H2(g) + ½O2(g) ——> H2O(g)
b) CH4(g) + 2O2(g) ——> CO2(g) + 2H2O(g)
c) H2(g) + Cl2(g) ——> 2HCl(g)
d) C2H5OH(g) + HBr(g) ——> C2H5Br(g) + H2O(g)
© KNOCKHARDY PUBLISHING 2009
6 F322 Chemical Energetics
Enthalpy change of reaction from enthalpy changes of combustion and formation
Formation If you formed the products from their elements you should need the same amounts
of every substance as if you formed the reactants from their elements.
By applying Hess’s Law ...
ELEMENTS
∆Ηr ∆Η f
A B
∆Η f
∆Ηf ∆Ηf
REACTANTS
∆Ηr
ELEMENTS
PRODUCTS
∆Ηr = Σ ∆Η f (PRODUCTS) Σ ∆Η f (REACTANTS)
example Calculate the standard enthalpy change for the following reaction, given that the
standard enthalpies of formation of water, nitrogen dioxide and nitric acid are -286,
+33 and -173 kJ mol-1 respectively. [oxygen’s value is ZERO as it is an element ]
2H2O(l) + 4NO2(g) + O2(g) ——> 4HNO3(l)
applying Hess’s Law ... ∆Η°r = [ 4(-173) ] - [ 2(-286) + 4(+33) + 0 ] = -252 kJ
Q.5 If the standard enthalpy changes of formation of SO2(g) and SO3(g) are
-296 and -395 kJ mol-1 respectively, calculate the enthalpy change of
reaction of ... 2SO2(g) + O2(g) ——> 2SO3(g)
© KNOCKHARDY PUBLISHING 2009
Chemical Energetics F322 7
Combustion If you burned all the products you should get the same amounts
of CO2 and H2O etc. as if you burned the reactants.
Applying Hess’s Law ...
REACTANTS
DH r
∆Ηr
A B
DHc
PRODUCTS
∆Ηc ∆Ηc
DHc
OXIDATION
OXIDATION
PRODUCTS PRODUCTS
∆Ηr = Σ ∆Η c (REACTANTS) Σ ∆Η c (PRODUCTS)
example Calculate the standard enthalpy change of formation of methane, given that the
standard enthalpies of combustion of carbon, hydrogen and methane are -394,
-286 and -890 kJ mol-1 respectively.
C(graphite) + 2H2(g) ——> CH4(g)
applying Hess’s law ... ∆Η°r = [ (-394) + 2(-286) ] - [ (-890) ] = -74 kJ mol-1
Q.6 Calculate the enthalpy change of reaction for H2 + C2H4 ——> C2H6
given that the enthalpy changes of combustion of H2, C2H4 and C2H6 are
-286, -1409 and -1560 kJ mol-1 respectively.
Compare this value with that obtained using average bond enthalpies.
© KNOCKHARDY PUBLISHING 2009
8 F322 Chemical Energetics
Measuring Enthalpy Changes
Calorimetry • involves the practical determination of enthalpy changes
• usually involves heating (or cooling) known amounts of water
water is heated up reaction is EXOTHERMIC
water cools down reaction is ENDOTHERMIC
Calculation The energy required to change the temperature of a substance can be found;
q = m x c x ∆Τ
where q = heat energy kJ
m = mass kg
c = Specific Heat Capacity kJ K -1 kg -1 [ water is 4.18 ]
∆Τ = change in temperature K
∆Τ The value of ∆Τ is usually calculated graphically by measuring the temperature
changes before, during and after a reaction.
Graphical method
★ ★ ★ ★
The temperature is taken every half ★ ★
★ ★
★ ★
★
minute before mixing the reactants.
Temperature / °C
Reactants are mixed after 3 minutes. ∆Τ
Further readings are taken every half
minute as the reaction mixture cools.
Extrapolate the lines as shown and
calculate the value of ∆Τ. ★ ★ ★ ★ ★
1 3 5 7 9
Time / minutes
Example 1 When 0.18g of hexane underwent complete combustion, it raised the temperature
of 100g (0.1kg) water from 22°C to 47°C. Calculate its enthalpy of combustion.
Heat absorbed by the water (q) = 0.1 x 4.18 x 25 = 10.45 kJ
Moles of hexane burned = mass / Mr = 0.18 / 86
= 0.00209
Enthalpy change = heat energy / moles = − 10.45 / 0.00209
ANS − 5000 kJ mol -1
© KNOCKHARDY PUBLISHING 2009
Chemical Energetics F322 9
Example 2 25cm3 of 2.0M HCl was added to 25cm3 of 2.0M NaOH in an insulated beaker.
The initial temperature of both solutions was 20°C. The reaction mixture was
stirred to ensure mixing and the highest temperature reached by the solution was
33°C. Calculate the Molar Enthalpy of Neutralisation.
Temperature rise (∆Τ) = 306K - 293K = 13K
Volume of resulting solution = 50cm3 = 0.05 dm3
Equivalent mass of water = 50g = 0.05 kg
Heat absorbed by the water (q) = 0.05 x 4.18 x 13 = 2.717 kJ
Moles of HCl reacting = 2 x 25/1000 = 0.05 mol
Moles of NaOH reacting = 2 x 25/1000 = 0.05 mol
Equation NaOH + HCl ——> NaCl + H2O
Moles of water produced = 0.05 mol
Enthalpy change per mol (∆Η) = − ( heat energy / moles of water )
= − 2.717 / 0.05
ANS − 54.34 kJ mol -1
Q.7 What is the usual value for the Molar Enthalpy of Neutralisation ?
Why might the value calculated from the reaction between sodium hydroxide and
ethanoic acid differ from the usual value?
Results from simple calorimetry experiments are seldom very accurate. Make a
list of possible sources of error and suggests improvements to the experiment.
© KNOCKHARDY PUBLISHING 2009
10 F322 Chemical Energetics
Enthalpy of Combustion of Alkanes
1. Write the equation representing the Standard Enthalpy Change of Combustion of heptane, C7H16.
..............................................................................................................................................
2. Using the data, plot a graph of Enthalpy of Combustion against number of carbon atoms.
Compound Enthalpy of Combustion / kJ mol-1
CH4 - 890
C2H6 - 1560
C3H8 - 2220
C4H10 - 2877
C5H12 - 3509
C6H14 - 4194
C8H18 - 5512
3. Use your graph to calculate the following
a) the value of the Enthalpy Change of Combustion of heptane ..........................
b) an approximate value for the Enthalpy Change of Combustion of hydrogen ..........................
4. State, giving reasons, any advantages of using butane as a household fuel.
.......................................................................................................................................................
.......................................................................................................................................................
.......................................................................................................................................................
5. State, giving reasons, any disadvantages of using butane as a household fuel.
.......................................................................................................................................................
.......................................................................................................................................................
.......................................................................................................................................................
6. Calculate the amount of heat produced when 1kg of the following undergo complete combustion.
a) CH4
b) C4H10
© KNOCKHARDY PUBLISHING 2009
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- 15 DeltahppDocument39 pages15 DeltahppSpam emailNo ratings yet
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsFrom EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsNo ratings yet
- Enthalpy, Hess Cycles and CalorimetryDocument10 pagesEnthalpy, Hess Cycles and CalorimetryHK Nova ChiuNo ratings yet
- DELTAHPPDocument39 pagesDELTAHPPapi-3706290No ratings yet
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- 6 - Enthalpy ChangeDocument28 pages6 - Enthalpy Changenamopoopp123456789No ratings yet
- ENTHALPYDocument38 pagesENTHALPYTeejay MakazhuNo ratings yet
- PPP Enthalpy ChangesDocument39 pagesPPP Enthalpy ChangesHenryLim9100% (1)
- Copia de As - Course Notes EnthalpypdfDocument18 pagesCopia de As - Course Notes Enthalpypdfmaria sanchezNo ratings yet
- Chem Energetics - ENTHALPY CHANGEDocument24 pagesChem Energetics - ENTHALPY CHANGEb972xmny46No ratings yet
- Ive + Ive: ThermodynamicsDocument10 pagesIve + Ive: ThermodynamicsAbbas HaiderNo ratings yet
- ER CH4-Student 2022Document38 pagesER CH4-Student 2022aboem16No ratings yet
- Ib Enthalpy KHDocument39 pagesIb Enthalpy KHSamer EhabNo ratings yet
- Chemical Energetics (As) 2023 With AnswersDocument12 pagesChemical Energetics (As) 2023 With AnswersNurli SallehNo ratings yet
- Chapter 5a (AS-Level) : Chemical EnergeticsDocument12 pagesChapter 5a (AS-Level) : Chemical EnergeticsMohamed AkkashNo ratings yet
- Gene Regulation - 2018Document4 pagesGene Regulation - 2018ArhanaNo ratings yet
- CH 5 Part 2 Bond Enthalpies and Hess LawDocument50 pagesCH 5 Part 2 Bond Enthalpies and Hess LawSafiye TerNo ratings yet
- Heat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherDocument12 pagesHeat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherAeyyjayyNo ratings yet
- NSSCAS Chemistry Theme 2 Topic 2.1 - Updated 21 October 2020Document48 pagesNSSCAS Chemistry Theme 2 Topic 2.1 - Updated 21 October 2020Peter KudumoNo ratings yet
- Chapter 6-Enthalpy ChangesDocument18 pagesChapter 6-Enthalpy ChangesClarize Soo Hoo0% (1)
- 5 Cie Chemical EnergeticsDocument17 pages5 Cie Chemical EnergeticsKhoai TâyNo ratings yet
- T8 - Energetics IDocument28 pagesT8 - Energetics II Kadek Irvan Adistha PutraNo ratings yet
- Chap 1 Chemical Reactions and EquationDocument48 pagesChap 1 Chemical Reactions and EquationArtesh Kumar 9bNo ratings yet
- Enthalpy ChangesDocument10 pagesEnthalpy ChangesPutri Nur SyafieqahNo ratings yet
- 9.1 Concept of Enthalpy PDFDocument32 pages9.1 Concept of Enthalpy PDFHaspreet GillNo ratings yet
- Chapter 34Document66 pagesChapter 34Nina FairuzNo ratings yet
- Chemical Equations & ReactionsDocument78 pagesChemical Equations & ReactionsIshvarya100% (1)
- Chapter 9 - Part 1Document63 pagesChapter 9 - Part 1muhammad izzul100% (1)
- Sheet - 01 - ThermochemistryDocument78 pagesSheet - 01 - ThermochemistrySushant VermaNo ratings yet
- Enthalpies of FormationDocument14 pagesEnthalpies of FormationAndrea BaduaNo ratings yet
- Chemical ReactionsDocument190 pagesChemical ReactionsAlbert Jade Pontimayor Legaria100% (1)
- Measuring Enthalpy ChangeDocument20 pagesMeasuring Enthalpy ChangedhruviniNo ratings yet
- Unit 4 Addition RXNDocument22 pagesUnit 4 Addition RXNSarewin RaviNo ratings yet
- Chemical Equations - ReactionsDocument41 pagesChemical Equations - ReactionsLegendNo ratings yet
- Energetics 1Document53 pagesEnergetics 1anellebrown299No ratings yet
- Chapter 1 - Thermochemistry PDFDocument87 pagesChapter 1 - Thermochemistry PDFannaNo ratings yet
- Chemical Reactions AnwersDocument164 pagesChemical Reactions AnwersAlbert Jade Pontimayor LegariaNo ratings yet
- Energetics - CN - STDT1Document2 pagesEnergetics - CN - STDT1NkemziNo ratings yet
- Introduction To Chemical Reactions and Equations: John Paul A. Reponte General Chemistry 1 STEM - Grade 11Document20 pagesIntroduction To Chemical Reactions and Equations: John Paul A. Reponte General Chemistry 1 STEM - Grade 11Hillary MarieNo ratings yet
- Enthalpy ChangesSMPLFDDocument55 pagesEnthalpy ChangesSMPLFDtopherchandraNo ratings yet
- Chemical Energetics - Bond EnthalpyDocument17 pagesChemical Energetics - Bond EnthalpyMunashe NyagonoNo ratings yet
- Chemical Reactions 1. Physical and Chemical Changes: - ReactantDocument2 pagesChemical Reactions 1. Physical and Chemical Changes: - ReactantNurmuhamed MasirdinovNo ratings yet
- Chapter 1 - ThermochemistryDocument87 pagesChapter 1 - ThermochemistryezanaNo ratings yet
- Topic 2 - ThermochemistryDocument25 pagesTopic 2 - Thermochemistrydeela decemberNo ratings yet
- 8 - Thermodynamics - Lecture 8Document19 pages8 - Thermodynamics - Lecture 8Ramy MaamounNo ratings yet
- Chem1010 Page l11 2023Document19 pagesChem1010 Page l11 2023Poowadon RattanasreethongNo ratings yet
- Energy Change in Chemical Reaction Alauddin Sir A & O Level Chemistry TeacherDocument8 pagesEnergy Change in Chemical Reaction Alauddin Sir A & O Level Chemistry TeacherMunshi LazimuzzamanNo ratings yet
- Chemical Equations & ReactionsDocument85 pagesChemical Equations & ReactionsEsther SparksNo ratings yet
- ElectrolyteDocument31 pagesElectrolyteJim tanNo ratings yet
- CHEM 1067 Lec 3 - 2019 - NJ - 4 PDFDocument21 pagesCHEM 1067 Lec 3 - 2019 - NJ - 4 PDFIbrahim AliNo ratings yet
- Chapter 1 CHM476 (Part 2)Document13 pagesChapter 1 CHM476 (Part 2)PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Chemical EnergeticsDocument34 pagesChemical EnergeticsNisidini JasingheNo ratings yet
- KIM 101E - Week 3 - BDocument70 pagesKIM 101E - Week 3 - Baliyasin200000No ratings yet
- CHEM1101 Worksheet 10: Enthalpy of Reaction ( ) Model 1: Endothermic and Exothermic ProcessesDocument4 pagesCHEM1101 Worksheet 10: Enthalpy of Reaction ( ) Model 1: Endothermic and Exothermic ProcessesJesus carbonoNo ratings yet
- GeneralChemistry 7Document34 pagesGeneralChemistry 7KIÊN ĐẶNG TRUNGNo ratings yet
- Lesson 3 - Chemical Reaction and EquationDocument48 pagesLesson 3 - Chemical Reaction and EquationJoanna Ruth SeproNo ratings yet
- 9 Thermochemistry EditedDocument108 pages9 Thermochemistry EditedNur AleyaNo ratings yet
- Grade 9 Angles Around A Point: Choose Correct Answer(s) From The Given ChoicesDocument3 pagesGrade 9 Angles Around A Point: Choose Correct Answer(s) From The Given ChoicesAmmar RizwanNo ratings yet
- Microscope: Dr. Hajra NazirDocument24 pagesMicroscope: Dr. Hajra NazirAmmar RizwanNo ratings yet
- By Dr. Hajra NazirDocument9 pagesBy Dr. Hajra NazirAmmar RizwanNo ratings yet
- Table of Diffusion, Osmosis & Active TransportDocument1 pageTable of Diffusion, Osmosis & Active TransportAmmar RizwanNo ratings yet
- Grade 9 Categorize Angles On The Basis of Their Measures: Choose Correct Answer(s) From The Given ChoicesDocument2 pagesGrade 9 Categorize Angles On The Basis of Their Measures: Choose Correct Answer(s) From The Given ChoicesAmmar RizwanNo ratings yet
- Effect of Exercise On BodyDocument10 pagesEffect of Exercise On BodyAmmar RizwanNo ratings yet
- AnglesDocument2 pagesAnglesAmmar RizwanNo ratings yet
- Fractions Worksheets For Kids - Printable Math Worksheets - Year 4 Math Worksheets - Vivax SolutionsDocument1 pageFractions Worksheets For Kids - Printable Math Worksheets - Year 4 Math Worksheets - Vivax SolutionsAmmar RizwanNo ratings yet
- Grade 9 Categorize Angles On The Basis of Their Measures: Choose Correct Answer(s) From The Given ChoicesDocument2 pagesGrade 9 Categorize Angles On The Basis of Their Measures: Choose Correct Answer(s) From The Given ChoicesAmmar RizwanNo ratings yet
- Evaluating One Variable Expressions: Evaluate Each Expression Using The Value GivenDocument2 pagesEvaluating One Variable Expressions: Evaluate Each Expression Using The Value GivenAmmar RizwanNo ratings yet
- Evaluating Two Variables ExpressionsDocument2 pagesEvaluating Two Variables ExpressionsAmmar RizwanNo ratings yet
- The Structure of Atoms2Document10 pagesThe Structure of Atoms2Zain KhanNo ratings yet
- Fractions Worksheets For Kids - Printable Math Worksheets - Year 4 Math Worksheets - Vivax Solutions2Document1 pageFractions Worksheets For Kids - Printable Math Worksheets - Year 4 Math Worksheets - Vivax Solutions2Ammar RizwanNo ratings yet
- Algebra Worksheets - Expanding Brackets - Year 8 Math Worksheets - Vivax Solutions4Document1 pageAlgebra Worksheets - Expanding Brackets - Year 8 Math Worksheets - Vivax Solutions4Ammar RizwanNo ratings yet
- Unit 1 Separation, Purification and Experimental Techniques PDFDocument10 pagesUnit 1 Separation, Purification and Experimental Techniques PDFAmmar RizwanNo ratings yet
- Algebra Worksheets - Expanding Brackets - Year 8 Math Worksheets - Vivax Solutions2Document1 pageAlgebra Worksheets - Expanding Brackets - Year 8 Math Worksheets - Vivax Solutions2Ammar RizwanNo ratings yet
- Algebra Worksheets - Expanding Brackets - Year 8 Math Worksheets - Vivax Solutions3Document1 pageAlgebra Worksheets - Expanding Brackets - Year 8 Math Worksheets - Vivax Solutions3Ammar RizwanNo ratings yet
- Absolute Value Equations: Math WorksheetsDocument2 pagesAbsolute Value Equations: Math WorksheetsAmmar RizwanNo ratings yet
- Algebra Expressions - Year 8 Math Worksheets - Vivax SolutionsDocument1 pageAlgebra Expressions - Year 8 Math Worksheets - Vivax SolutionsAmmar RizwanNo ratings yet
- Algebra Worksheets - Expanding Brackets - Year 8 Math Worksheets - Vivax SolutionsDocument1 pageAlgebra Worksheets - Expanding Brackets - Year 8 Math Worksheets - Vivax SolutionsAmmar RizwanNo ratings yet
- Transition MetalDocument3 pagesTransition MetalZain KhanNo ratings yet
- Tit RationDocument1 pageTit RationZain KhanNo ratings yet
- Chapter 1 - Experimental ChemistryDocument5 pagesChapter 1 - Experimental ChemistryQuan YingNo ratings yet
- Algebra Expressions - Year 8 Math Worksheets - Vivax Solutions2Document1 pageAlgebra Expressions - Year 8 Math Worksheets - Vivax Solutions2Ammar RizwanNo ratings yet
- Atoms: The Structure of AtomsDocument5 pagesAtoms: The Structure of AtomsZhafirul ZamanhuriNo ratings yet
- Thermodynamics 2Document10 pagesThermodynamics 2Zain KhanNo ratings yet
- The Petrochemical IndustryDocument3 pagesThe Petrochemical IndustryAbbas HaiderNo ratings yet
- Salt Preparations and Making Crystals PDFDocument2 pagesSalt Preparations and Making Crystals PDFAmmar RizwanNo ratings yet
- Solutions Solubilities PDFDocument4 pagesSolutions Solubilities PDFAmmar RizwanNo ratings yet
- Drug Education: September 9, 2017 Mati Davao OrientalDocument119 pagesDrug Education: September 9, 2017 Mati Davao OrientalYem Binobo NantesNo ratings yet
- Enantiomers Evaluation CetirizineDocument4 pagesEnantiomers Evaluation Cetirizinebebel555No ratings yet
- Oxidation NumberDocument21 pagesOxidation NumberChristian LopezNo ratings yet
- Poster Carica PDFDocument1 pagePoster Carica PDFBimo A.SNo ratings yet
- Boas Práticas para Produção de ADBlueDocument27 pagesBoas Práticas para Produção de ADBluewelyson_henriqueNo ratings yet
- Evaporation-An IntroductionDocument23 pagesEvaporation-An IntroductionKusmakarNo ratings yet
- Waterborne Acrylic Primer Nacorr Synergy With Halox SZP-391: Formulation Ci-101Document2 pagesWaterborne Acrylic Primer Nacorr Synergy With Halox SZP-391: Formulation Ci-101Swapnil AlandNo ratings yet
- Benzocaine Synthesis PDFDocument2 pagesBenzocaine Synthesis PDFLive FlightsNo ratings yet
- Drain Cleaner: Safety Data SheetDocument5 pagesDrain Cleaner: Safety Data SheetSuresh SubbuNo ratings yet
- LK Priceguide2003Document24 pagesLK Priceguide2003Praveen PrabhakaranNo ratings yet
- Valuable CattleDocument2 pagesValuable CattleGurmeet BrarNo ratings yet
- LDP200 Series DatasheetDocument6 pagesLDP200 Series DatasheetReza RamadhanNo ratings yet
- Iso 14687 3 2014Document11 pagesIso 14687 3 2014Tatiana Sainara Maia FernandesNo ratings yet
- Tooth DecayDocument28 pagesTooth DecayRyan Carlo CondeNo ratings yet
- Refrigeration Cycle, HVAC System Basics and Refrigerant Charging PDFDocument13 pagesRefrigeration Cycle, HVAC System Basics and Refrigerant Charging PDFMurillo MendesNo ratings yet
- Soda AshDocument10 pagesSoda Ashdr chatti hanumantha rao0% (1)
- Biomechanical Properties of A New Fiber-Reinforced CompositesDocument10 pagesBiomechanical Properties of A New Fiber-Reinforced Compositesazam ahmedNo ratings yet
- AntiepilepticiDocument29 pagesAntiepilepticiIskraNo ratings yet
- InternshipDocument16 pagesInternshipSarthak SinghNo ratings yet
- Manual Overlay WeldingDocument8 pagesManual Overlay Weldingcarlmac6183% (6)
- Ray Bowl MillDocument9 pagesRay Bowl MillAnup MinjNo ratings yet
- Valves SpecificationDocument13 pagesValves Specificationkselvan_1No ratings yet
- WPS - PQR (Sa516 GR.70)Document4 pagesWPS - PQR (Sa516 GR.70)miltonangulomorrisNo ratings yet
- Fick Second LawDocument9 pagesFick Second LawJohnny WoodsNo ratings yet
- Nafees Nastaleeq v1.02Document2 pagesNafees Nastaleeq v1.02latifshaikh20No ratings yet
- Detailed Storage Tank SizingDocument18 pagesDetailed Storage Tank SizingBooLat Johorean100% (3)
- (2017) Toxicological Effects of Glycyrrhiza Glabra (Licorice) A ReviewDocument16 pages(2017) Toxicological Effects of Glycyrrhiza Glabra (Licorice) A ReviewicaNo ratings yet
- Householders Guide To Flat RoofingDocument24 pagesHouseholders Guide To Flat RoofingBudi SudrajatNo ratings yet
- National Waste Management Strategy 2019-2023Document64 pagesNational Waste Management Strategy 2019-2023Chikondi KanamaNo ratings yet
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Guidelines for Integrating Process Safety into Engineering ProjectsFrom EverandGuidelines for Integrating Process Safety into Engineering ProjectsNo ratings yet
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet