Professional Documents
Culture Documents
U-43 IC Application Note No.: Title: Assay Determination of Cefadroxil According To USP
U-43 IC Application Note No.: Title: Assay Determination of Cefadroxil According To USP
Uploaded by
Khanza26Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
U-43 IC Application Note No.: Title: Assay Determination of Cefadroxil According To USP
U-43 IC Application Note No.: Title: Assay Determination of Cefadroxil According To USP
Uploaded by
Khanza26Copyright:
Available Formats
IC Application Note No.
U-43
Title: Assay determination of Cefadroxil according
to USP
Summary: Determination of Cefadroxil according to USP 28-NF 23
(second supplement) using RP chromatography with UV
detection.
Sample: Cefadroxil tablets
Sample Preparation: One tablet is dissolved in 1 L ultrapure water, injection
after subsequent filtration (0.45 µm) and 1:5 dilution
Column: 6.1008.100 Prontosil 120-5-C18 AQ, 150 mm
Wavelength: 230 nm
Eluent: Phosphate buffer pH = 5.0 / acetonitrile (96:4)
Flow: 1.5 mL/min
Injection Volume: 10 µL
mAU
Cefadroxil
1500
1000
500
Abs.1
0 1 2 3 4 min
Chromatogram: Cefadroxil
mg/mL
0.199
Results:
Test factors Value Limit Result
Theoretical plates 5577 More than 1800 PASS
Tailing factor 1.2 Less than 2.2 PASS
RSD (%, n = 3) 0.4 Less than 2.0 PASS
U-43
You might also like
- CVUA Karlsruhe Method Based On UHPLC-APCI-MS - MSDocument7 pagesCVUA Karlsruhe Method Based On UHPLC-APCI-MS - MSDip DuttaNo ratings yet
- Oat HPLC 4Document11 pagesOat HPLC 4mahatir muhammadNo ratings yet
- Official: Levo Oxacin TabletsDocument10 pagesOfficial: Levo Oxacin TabletsArmira AlexanderNo ratings yet
- 0015 Pharmaceutical Impurity Profiling Esomeprazole Magnesium MKDocument1 page0015 Pharmaceutical Impurity Profiling Esomeprazole Magnesium MKCECILIA PNo ratings yet
- Hydrolytic Degradation Profiling of Ezetimibe by HPLC MethodDocument6 pagesHydrolytic Degradation Profiling of Ezetimibe by HPLC MethodTJPRC PublicationsNo ratings yet
- Polynuclear Aromatic Hydrocarbons by HPLCDocument9 pagesPolynuclear Aromatic Hydrocarbons by HPLCLuis VilchezNo ratings yet
- Acid Phosphatase α-Naphtylphosphate. Kinetic.: 19 x 2 mL. Ref.: 30110 Store: 2 - 8 ºCDocument1 pageAcid Phosphatase α-Naphtylphosphate. Kinetic.: 19 x 2 mL. Ref.: 30110 Store: 2 - 8 ºCMeilindaDwiLestariNo ratings yet
- Fluorescente LEDDocument1 pageFluorescente LEDWalter Olivari RamirezNo ratings yet
- Hydrolytic Degradation Profiling of Ezetimibe by HPLC MethodDocument6 pagesHydrolytic Degradation Profiling of Ezetimibe by HPLC MethodTJPRC PublicationsNo ratings yet
- 17 Resultant DiscussionDocument35 pages17 Resultant Discussionvarsha02jadhavNo ratings yet
- USP-NF ArginineDocument2 pagesUSP-NF ArginineIVAN BERNALNo ratings yet
- K. Geenens, N. Clottens, V. Vergote, D. Coucke, E. Mehuys and B. de SpiegeleerDocument1 pageK. Geenens, N. Clottens, V. Vergote, D. Coucke, E. Mehuys and B. de SpiegeleerIna LabokNo ratings yet
- Aromatic Amino AcidsDocument2 pagesAromatic Amino Acidsait el hocine tarekNo ratings yet
- High Resolution Mass SpectrometryDocument26 pagesHigh Resolution Mass SpectrometryJoseFernandoLozanoDuranNo ratings yet
- Automated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Fibrinogen)Document8 pagesAutomated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Fibrinogen)Aahsan Iqbal احسن اقبالNo ratings yet
- Automated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Fibrinogen)Document8 pagesAutomated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Fibrinogen)Aahsan Iqbal احسن اقبالNo ratings yet
- Automated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Fibrinogen)Document8 pagesAutomated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Fibrinogen)Aahsan Iqbal احسن اقبالNo ratings yet
- Automated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Fibrinogen)Document8 pagesAutomated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Fibrinogen)Aahsan Iqbal احسن اقبالNo ratings yet
- AN 259 IC N Methylpyrrolidine Cefepime LPN2586 ENDocument6 pagesAN 259 IC N Methylpyrrolidine Cefepime LPN2586 ENDewi WulandhariNo ratings yet
- Bisoprolol Fumarate Tablets USPDocument1 pageBisoprolol Fumarate Tablets USPFelix PrawiraNo ratings yet
- Whmis Material Safety Data Sheet: 1. Chemical Product and Company IdentificationDocument6 pagesWhmis Material Safety Data Sheet: 1. Chemical Product and Company IdentificationSimonNo ratings yet
- RP-HPLC Method Development and Validation For Simultaneous Estimation of Amlodipine and Atenolol and Pharmaceutical Dosage FormDocument34 pagesRP-HPLC Method Development and Validation For Simultaneous Estimation of Amlodipine and Atenolol and Pharmaceutical Dosage FormLikithaNo ratings yet
- Hcys - HPLC KitDocument2 pagesHcys - HPLC Kitait el hocine tarekNo ratings yet
- Benzotriazole Tolytriazole AP 74 900Document4 pagesBenzotriazole Tolytriazole AP 74 900wulalan wulanNo ratings yet
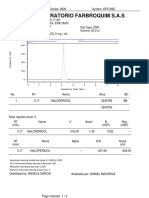
- Laboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Document3 pagesLaboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Brendapaez3No ratings yet
- General Information IUPAC Name: CFR: CAS #: Synonyms: Source: Appearance: Kovat's Index: UV (NM)Document6 pagesGeneral Information IUPAC Name: CFR: CAS #: Synonyms: Source: Appearance: Kovat's Index: UV (NM)anastasiaNo ratings yet
- Acebutolol Hydrochloride Capsules - USPDocument2 pagesAcebutolol Hydrochloride Capsules - USPДарія ОсадчаNo ratings yet
- U-11 IC Application Note No.: Title: N-Acetylcysteine in Medication Against Sinusitis According To USPDocument2 pagesU-11 IC Application Note No.: Title: N-Acetylcysteine in Medication Against Sinusitis According To USPKhanza26No ratings yet
- Naproxen TabletDocument2 pagesNaproxen TabletJunaid ZafarNo ratings yet
- NIOSH 6011 HalidaDocument6 pagesNIOSH 6011 HalidaAmalia NurbandiniNo ratings yet
- Norwegian-Latvian Research Cooperation Activity: EEA/Norway Grants 2009-2014 Programme LV05Document27 pagesNorwegian-Latvian Research Cooperation Activity: EEA/Norway Grants 2009-2014 Programme LV05ivan cuadradoNo ratings yet
- 040a2h65na Lab enDocument1 page040a2h65na Lab enIda Bagus MahendraNo ratings yet
- U-34 IC Application Note No.: Title: Chromatographic Purity Determination of Ibuprofen According To USPDocument1 pageU-34 IC Application Note No.: Title: Chromatographic Purity Determination of Ibuprofen According To USPolga_bravo_19No ratings yet
- Automated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Thrombin Time)Document10 pagesAutomated Blood Coagulation Analyzer CA-50: Basic Data Sheet - (Thrombin Time)Aahsan Iqbal احسن اقبالNo ratings yet
- Acebutolol Hydrochloride CapsulesDocument2 pagesAcebutolol Hydrochloride CapsulesRaquel BcNo ratings yet
- JWH018Document6 pagesJWH018GenceNo ratings yet
- GUID - 2 en-USDocument2 pagesGUID - 2 en-USDilawar BakhtNo ratings yet
- BD Facscanto Ii Flow Cytometer: Technical SpecificationsDocument4 pagesBD Facscanto Ii Flow Cytometer: Technical Specificationsابكر ابو ميلادNo ratings yet
- USP-NF Acepromazine Maleate InjectionDocument2 pagesUSP-NF Acepromazine Maleate InjectionStalin VacaNo ratings yet
- TOC Fusion For Purified and WFI - JPDocument5 pagesTOC Fusion For Purified and WFI - JPPrianurraufikachmadNo ratings yet
- Titulacion Fotometrica MnSO4 - MethromDocument2 pagesTitulacion Fotometrica MnSO4 - MethromKeila ChavesNo ratings yet
- USP-NF Acebutolol Hydrochloride CapsulesDocument4 pagesUSP-NF Acebutolol Hydrochloride CapsulesStalin VacaNo ratings yet
- 2,5-Dimethoxy-4-Iodophenethylamine: 1. Synonyms CFR: CAS #Document11 pages2,5-Dimethoxy-4-Iodophenethylamine: 1. Synonyms CFR: CAS #Strejtoje ČistunovićNo ratings yet
- Alendronate in Tablets According To The Chinese PharmacopeiaDocument2 pagesAlendronate in Tablets According To The Chinese PharmacopeiaKhanza26No ratings yet
- PKI - AN - 2016 - Analysis of Oil Additives Following ASTM D4951 With The Avio 200 ICPOESDocument3 pagesPKI - AN - 2016 - Analysis of Oil Additives Following ASTM D4951 With The Avio 200 ICPOESstrubingeraNo ratings yet
- Problem Set 13 and 14Document6 pagesProblem Set 13 and 14sophia del rosario100% (1)
- Test Method For The Determination of NDMA by LC/MS/MS in Valsartan Finished ProductsDocument7 pagesTest Method For The Determination of NDMA by LC/MS/MS in Valsartan Finished ProductsAki FuyuNo ratings yet
- Quantitative Amino AcidsDocument4 pagesQuantitative Amino Acidsait el hocine tarekNo ratings yet
- Test Results YLS-3000-K SN R19051602 IPG (Beijing) Fiber Laser Technology Co., Ltd.Document13 pagesTest Results YLS-3000-K SN R19051602 IPG (Beijing) Fiber Laser Technology Co., Ltd.autista24No ratings yet
- Ferric Reducing Antioxidant Power (FRAP) Assay Kit (Colorimetric)Document2 pagesFerric Reducing Antioxidant Power (FRAP) Assay Kit (Colorimetric)jayven minguillanNo ratings yet
- Analisis Morfin Dan Opiat GC-MSDocument3 pagesAnalisis Morfin Dan Opiat GC-MSintan kusumaningtyasNo ratings yet
- Review Article On Sumatriptan Plus Promethazine Validation Methods.Document10 pagesReview Article On Sumatriptan Plus Promethazine Validation Methods.Nazneen patelNo ratings yet
- Alprazolam Extended-Release TabletsDocument5 pagesAlprazolam Extended-Release TabletsRaquel BcNo ratings yet
- Application Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerDocument4 pagesApplication Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerPrianurraufikachmadNo ratings yet
- USP-NF AdenineDocument3 pagesUSP-NF AdenineWillian SilvaNo ratings yet
- Equ211-01 Siemens Dimension Fast FactsDocument26 pagesEqu211-01 Siemens Dimension Fast Factsmagendi indra muktiNo ratings yet
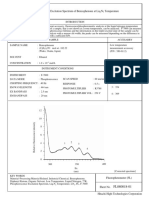
- Phosphorescence Excitation Spectrum of Benzophenone at Liq.N TemperatureDocument5 pagesPhosphorescence Excitation Spectrum of Benzophenone at Liq.N TemperatureNisar Ali Mphil-Chem ANo ratings yet
- GC - Shimadzu GCMS Terpene ApplicationDocument9 pagesGC - Shimadzu GCMS Terpene ApplicationpilarNo ratings yet
- GUID - 4 en-USDocument1 pageGUID - 4 en-USDilawar BakhtNo ratings yet
- Applications of Zeeman Graphite Furnace Atomic Absorption Spectrometry in the Chemical Laboratory and in ToxicologyFrom EverandApplications of Zeeman Graphite Furnace Atomic Absorption Spectrometry in the Chemical Laboratory and in ToxicologyC. MinoiaNo ratings yet
- Standard Test Method For Anion in Caustric SodaDocument5 pagesStandard Test Method For Anion in Caustric SodaKhanza26No ratings yet
- Test For Any of The Following ContaminantsDocument1 pageTest For Any of The Following ContaminantsKhanza26No ratings yet
- Fast Facts: Inline Dialysis For Professional IC Instruments 01Document2 pagesFast Facts: Inline Dialysis For Professional IC Instruments 01Khanza26No ratings yet
- Alendronate in Tablets According To The Chinese PharmacopeiaDocument2 pagesAlendronate in Tablets According To The Chinese PharmacopeiaKhanza26No ratings yet
- U-11 IC Application Note No.: Title: N-Acetylcysteine in Medication Against Sinusitis According To USPDocument2 pagesU-11 IC Application Note No.: Title: N-Acetylcysteine in Medication Against Sinusitis According To USPKhanza26No ratings yet
- Fast Facts: Inline Ultrafiltration For Professional IC Instruments 01Document2 pagesFast Facts: Inline Ultrafiltration For Professional IC Instruments 01Khanza26No ratings yet
- Combustion IC PDFDocument12 pagesCombustion IC PDFKhanza26No ratings yet
- Which Methode For Bromate Analysis PDFDocument3 pagesWhich Methode For Bromate Analysis PDFKhanza26No ratings yet
- Which Methode For Bromate Analysis PDFDocument3 pagesWhich Methode For Bromate Analysis PDFKhanza26No ratings yet