Professional Documents
Culture Documents
Heat Transfer When T 0
Heat Transfer When T 0
Uploaded by
kumar0 ratings0% found this document useful (0 votes)
32 views2 pagesOriginal Title
Heat transfer when ∆t=0
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
32 views2 pagesHeat Transfer When T 0
Heat Transfer When T 0
Uploaded by
kumarCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
What does ∆t=0 mean for a heat transfer between two closed systems? Steady state?
State of thermal
equilibrium? Isenthalpic equilibrium? Constant entropy? Thermodynamic equilibrium? What?
Let us imagine, two thermal reservoirs A and B are typical two closed systems connected by a path
which allows energy transfer but not the material transfer across the boundary of the system. Let us
examine, what does heat transfer mean for a closed system, when ∆t=0 between two reservoirs.
Two key definitions:
Closed system: In thermodynamics, a closed system can exchange energy (as heat or work) but not
matter, with its surroundings.
Temperature: Temperature is a physical property of matter that quantitatively expresses hot and cold. It
is a measure of the ability of a substance, or more generally of any physical system, to transfer heat
energy to another physical system. The temperature of a substance is closely related to the average
kinetic energy of its molecules.
Let us evaluate A and B
Given, A and B are two closed systems connected by path permeable to heat and energy but not material
transfer with ∆t=0
An important point to note is that when ∆t=0, while externally, there is no heat transfer between A and B
heat exchange does not stop internally, both A and B are in state of dynamic equilibrium. In A and B
molecules have variable kinetic energies, they are in motion with different kinetic energies, and pressure,
therefore, chances of a closed system internally being in a state of equilibrium is just impossible.
Are A and B in steady state ?
No. Steady state is a condition of system if the state variables which define the behaviour of the system or
the process do not change with time. In thermodynamics, a state variable is an independent variable of a
state function like internal energy, enthalpy, and entropy. Examples include temperature, pressure, and
volume.
A steady state wants three variables temperature [T], pressure [P] and volume [V] to remain unchanged
with time. Therefore, just ∆t=0 does not mean that A and B are in conditions of steady state.
Are A and B in thermal equilibrium?
Yes. Thermal equilibrium is a condition if there is no net flow of thermal energy between them when they
are connected by a path permeable to heat.
QA = Mass A x Specific heat A x Temperature A
QB = Mass B x Specific heat B x Temperature B
When ∆t = 0, Temperature A = Temperature B, it implies that [Mass A x Specific heat A <= > Mass B x
Specific heat B] are in dynamic equilibrium. Temperature is output of heat that takes into account
temperature rise per unit mass of fluid depending on the specific heat of the fluid. Therefore, when ∆t =
0, Q A < = > Q B, are in thermal equilibrium. Equal amount of heat exchange is still taking place
between A and B. Internally both A and B are in still in thermally dynamic state. Q is an energy in
transit. It does not belong to either A or B. It just moves from high to low temperature. It may however be
noted while product of mass and specific heat is identical in A and B, individually not necessarily they are
same. Therefore, when A and B have ∆t = 0 , though they are in thermal equilibrium, internally, their
molecules can have completely different kinetic energy and entropy and energy loses.
Are A and B in an isenthalpic state ?
No. Isenthalpic state is a process in which both systems have same energy, ∆H= 0. Every material has
enthalpy of formation, which is energy required to create that material when it was formed.
∆H = ∆U + P∆V [ H, U , P, and V are enthalpy, internal energy, pressure and volume respectively]. P∆V
signifies work energy. First point to be noted is enthalpy is sum of internal energy and work energy. A
closed system is permeable for work energy transfer in – out to surrounding. When the system loses
energy in the process of work energy exchange, it gets subtracted from its enthalpy, H When the system
gains energy in the process of work energy exchange, it gets added to its enthalpy, H. For a process to be
isenthalpic , the sum of [∆U + P∆V] needs to be zero. At ∆t=0 , only ∆Q is zero and not [∆U + P∆V] ∆Q is
just one part of energy, [∆U + P∆V] and not total energy. Therefore just, ∆t=0 does not make a closed
system isenthalpic.
Are A and B in a state of constant entropy?
No. In thermodynamics, an isentropic process is both adiabatic and reversible. It needs no transfer of heat
or matter which is possible only in isolated system. A closed system has limitation, it is permeable to heat
transfer. Therefore, A and B as closed systems can’t be in a state of isentropy, ∆S=0 [S is entropy] .
Are A and B In thermodynamic equilibrium?
No, a closed system is permeable to energy and therefore, closed system cannot produce a state of
thermodynamic equilibrium. For a thermodynamic equilibrium to establish you need an isolated system
which is impermeable to energy and material transfer.
What is thermodynamic equilibrium?
Thermodynamic equilibrium is a concept of thermodynamics which requires all state variables like
internal energy, enthalpy, entropy are internally and externally in equilibrium in an isolated system, In
other words, there is no driving force to make any macroscopic change in the system. In terms of Gibbs
free energy change, ∆G = ∆H -T∆S = 0, In other words, when Gibbs free energy change, ∆G internally in a
system is zero, it has no free energy to carryout any macroscopic change in the system.
You might also like
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5813)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (844)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- 3 - A Detailed Lesson Plan in Science XDocument12 pages3 - A Detailed Lesson Plan in Science XRanas, Matthew T.No ratings yet
- Multiple Choice ReviewerDocument3 pagesMultiple Choice ReviewerVeronicaNo ratings yet
- Regenerative Braking System in AutomobilDocument2 pagesRegenerative Braking System in AutomobilAryan AmeenNo ratings yet
- General Biology Midterm Exam: Chapter 1: Introduction - Themes in The Study of LifeDocument4 pagesGeneral Biology Midterm Exam: Chapter 1: Introduction - Themes in The Study of LifeNermaDžaferovićNo ratings yet
- HW 4 3.12Document33 pagesHW 4 3.12Anonymous U3DpVvqVWx0% (3)
- National Institute of Industrial Engineering: NITIE MumbaiDocument2 pagesNational Institute of Industrial Engineering: NITIE MumbaikumarNo ratings yet
- TATA Steel JET Syllabus 2020 PDF - Download Junior Engineer Trainee Exam PatternDocument8 pagesTATA Steel JET Syllabus 2020 PDF - Download Junior Engineer Trainee Exam PatternkumarNo ratings yet
- Mechanical MetallurgyDocument46 pagesMechanical MetallurgykumarNo ratings yet
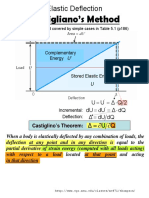
- Castigliano's Method: Elastic DeflectionDocument8 pagesCastigliano's Method: Elastic DeflectionkumarNo ratings yet
- Electron Beam WeldingDocument20 pagesElectron Beam WeldingkumarNo ratings yet
- AMORPHOUS and CRYSTALLINE SOLIDSDocument5 pagesAMORPHOUS and CRYSTALLINE SOLIDSJust PatriciaNo ratings yet
- VIKRAM training-REPORT PDFDocument30 pagesVIKRAM training-REPORT PDFdixowo6103No ratings yet
- Comparison Chart Led Lights vs. Incandescent Light Bulbs vs. CflsDocument6 pagesComparison Chart Led Lights vs. Incandescent Light Bulbs vs. CflsPankaj ShidurkarNo ratings yet
- Al6061 With Bamboo CharDocument11 pagesAl6061 With Bamboo CharLuisNo ratings yet
- Twinkling of StarsDocument2 pagesTwinkling of StarsVikash SharmaNo ratings yet
- NFPA DiamondDocument17 pagesNFPA DiamondEurek Mago LumaguiNo ratings yet
- Final TBL Group 8 Section ADocument11 pagesFinal TBL Group 8 Section AGaayaatrii BehuraaNo ratings yet
- Aset 3d Mapping Tool Alvarez 1Document2 pagesAset 3d Mapping Tool Alvarez 1api-530275600No ratings yet
- Solar Thermal PowerDocument30 pagesSolar Thermal PowerMd Moinul Alom ShovonNo ratings yet
- Power Factor CorrectionDocument4 pagesPower Factor CorrectionimfaroshaNo ratings yet
- Cosmology and The Birth of EarthDocument34 pagesCosmology and The Birth of EarthTUMELO MATLHABANo ratings yet
- Ecological Economics: Jampel Dell'Angelo, Maria Cristina Rulli, Paolo D'OdoricoDocument10 pagesEcological Economics: Jampel Dell'Angelo, Maria Cristina Rulli, Paolo D'OdoricoMatheus Basso RomanNo ratings yet
- CompactionDocument100 pagesCompactionVaibhav PatilNo ratings yet
- EPS Insulation EPDDocument18 pagesEPS Insulation EPDmadyaNo ratings yet
- Chemistry Heating-Cooling MathDocument14 pagesChemistry Heating-Cooling MathM Carlson0% (1)
- Student Exploration: Phases of WaterDocument5 pagesStudent Exploration: Phases of WaterGabriel LouimaNo ratings yet
- CBE 547 Course Outline (Syllabus)Document1 pageCBE 547 Course Outline (Syllabus)teerth_brahmbhatt100% (1)
- CE 355 Unit 4Document27 pagesCE 355 Unit 4Nsb JejsNo ratings yet
- Chapter 5: ThermochemistryDocument22 pagesChapter 5: ThermochemistryHakimNo ratings yet
- STP Gas Calculations PracticeDocument22 pagesSTP Gas Calculations PracticeKen IlgenfritzNo ratings yet
- Soil ResourcesDocument17 pagesSoil ResourcesEji AlcorezaNo ratings yet
- A Pilot Recycling of Plastic Pure Water Sachets/Bottles Into Composite Floor Tiles: A Case Study From Selected Dumping Site in OgbomosoDocument6 pagesA Pilot Recycling of Plastic Pure Water Sachets/Bottles Into Composite Floor Tiles: A Case Study From Selected Dumping Site in OgbomosoVisaya L. ClaudineNo ratings yet
- How Primary Water Can Solve The Global Water CrisisDocument76 pagesHow Primary Water Can Solve The Global Water CrisisDebraNo ratings yet
- Center of MassDocument13 pagesCenter of MassFrancisQuiwaNo ratings yet
- Much Needed Electricity For Palawan: Relocated Power SuppliesDocument2 pagesMuch Needed Electricity For Palawan: Relocated Power SuppliesEana Jamaica MadriñanNo ratings yet