Professional Documents
Culture Documents
Potential Pathogenesis and Clinical Aspects of Pulmonary Fibrosis Associated With Rheumatoid Arthritis
Uploaded by
Liya SuwarniOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Potential Pathogenesis and Clinical Aspects of Pulmonary Fibrosis Associated With Rheumatoid Arthritis
Uploaded by
Liya SuwarniCopyright:
Available Formats
Potential Pathogenesis and Clinical Aspects of
Pulmonary Fibrosis Associated with
Rheumatoid Arthritis
BERNADETTE R. GOCHUICO, MD
ABSTRACT: Pulmonary fibrosis is an extra-articular dis- opment of an altered immunologic response, and/or
order that can occur in association with rheumatoid aberrant host repair processes. The clinical course of
arthritis. The differential diagnosis of this disorder is patients with pulmonary fibrosis and rheumatoid arthri-
similar to that of idiopathic pulmonary fibrosis, but tis is heterogeneous but is generally insidious, chronic,
specific entities such as atypical pulmonary infections and progressive. These patients respond unpredictably
and drug-induced interstitial lung disease must be con- to available empiric therapeutic agents and, overall,
sidered as causes of pulmonary fibrosis in patients with their prognosis is poor; limited data suggests that the
rheumatoid arthritis. Although the cause of lung fibrosis median survival time can be less than 4 years. KEY
in persons with rheumatoid arthritis is unknown, factors INDEXING TERMS: Pulmonary fibrosis; Interstitial lung
that can potentially contribute to the pathogenesis of this disease; Lung; Rheumatoid arthritis; Collagen vascular
pulmonary disease include genetic susceptibility, devel- disease. [Am J Med Sci 2001;321(1):83–88.]
associated with various pulmonary abnormalities1,2.
P ulmonary fibrosis is an extra-articular disorder
that can occur in association with rheumatoid
arthritis.1,2 The differential diagnosis of this disor-
Pulmonary manifestations of rheumatoid arthritis
include pulmonary fibrosis, pleural disease, lung
der is similar to that of idiopathic pulmonary fibro- nodules, Caplan syndrome, bronchiolitis obliterans
sis, but specific entities such as atypical pulmonary with or without organizing pneumonia, bronchiecta-
infections and drug-induced interstitial lung disease sis, and pulmonary vasculitis (Table 1).2 In addition,
must be considered as causes of pulmonary fibrosis lung disease in patients with rheumatoid arthritis
in patients with rheumatoid arthritis.3–11 Although can be caused by infections or therapeutic agents
the cause of lung fibrosis in persons with rheumatoid commonly used to treat rheumatoid arthritis, such
arthritis is unknown, factors that can potentially con- as methotrexate and gold salts.3–11 Although some of
tribute to the pathogenesis of this pulmonary disease these pulmonary disorders are benign and asymp-
include genetic susceptibility, development of an al- tomatic, the development of fibrotic lung disease is
tered immunologic response, and/or aberrant host re- notable because it is one of the leading causes of
pair processes.12–18 The clinical course of patients with death in these patients, irrespective of therapy.2
pulmonary fibrosis and rheumatoid arthritis is heter- Reports of epidemiologic studies have estimated
ogeneous but is generally insidious, chronic, and pro- the prevalence of pulmonary fibrosis to be between 1
gressive.1 These patients respond unpredictably to and 58% in patients with rheumatoid arthritis. This
available empiric therapeutic agents and, overall, wide variability is caused by the use of inconsistent
their prognosis is poor; limited data suggests that the diagnostic criteria and detection methods for pulmo-
median survival time can be less than 4 years.1,2,19 nary fibrosis in published studies. For example,
studies that based the diagnosis on chest roentgen-
Epidemiology ographic findings report an estimated prevalence of
fibrotic lung disease at 1 to 12%, whereas those that
Rheumatoid arthritis is a systemic disorder affect-
ing 0.5 to 2% of the general population and can be established the diagnosis using reduced diffusion
capacity or other pulmonary physiology data report
an estimated prevalence of about 41% of patients
From the Pulmonary-Critical Care Medicine Branch, National with rheumatoid arthritis.20,21 When multiple mea-
Heart, Lung, and Blood Institute, National Institutes of Health, sures, such as bronchoalveolar lavage (BAL) cell
Bethesda, MD 20892.
Correspondence: PCCMB-NHLBI, NIH, Building 10, Room
counts, chest radiographs, high-resolution computed
6D03, 10 Center Drive MSC 1590, Bethesda, MD 20892-1590 tomography (HRCT), and 99m-Technetium diethyl-
(E-mail: gochuicb@nhlbi.nih.gov). ene triamine pentaacetate (99m-TcDTPA) radionu-
THE AMERICAN JOURNAL OF THE MEDICAL SCIENCES 83
Pulmonary Fibrosis Associated with Rheumatoid Arthritis
Table 1. Pulmonary Diseases Associated with Rheumatoid Table 2. Differential Diagnosis of Pulmonary Fibrosis
Arthritis Associated with Rheumatoid Arthritis
Pulmonary fibrosis Pulmonary fibrosis secondary to other collagen vascular
Pleural disease disorders
Pleural effusion Systemic sclerosis
Pleural thickening Systemic lupus erythematosus
Lung nodules Polymyositis
Caplan syndrome Dermatomyositis
Bronchiolitis obliterans Sjogren syndrome
Bronchiolitis obliterans with organizing pneumonia Mixed connective tissue disease
Bronchiectasis Pulmonary infections
Pulmonary vasculitis Mycobacteria species (especially M tuberculosis)
Pneumocystis carinii
Cytomegalovirus
Medication-induced lung disease
clide lung clearance scanning were used in evaluat- Methotrexate
Gold compounds
ing persons newly diagnosed with rheumatoid Sulfasalazine
arthritis, abnormalities consistent with interstitial D-penicillamine
lung disease were recognized in 58% of these pa- Granulomatous lung disease
tients.22 Thus, the prevalence of interstitial lung Sarcoidosis
disease in patients with rheumatoid arthritis may Langerhans cell granulomatosis
Chronic hypersensitivity pneumonitis
have been historically underestimated by studies Inhaled particle-induced fibrosis
that employed insensitive methods for detecting this Asbestos
disorder. Silica
Beryllium
Coal
Differential Diagnosis Radiation pneumonitis
Recurrent aspiration pneumonitis
The differential diagnosis of pulmonary fibrosis Amyloidosis
with or without associated rheumatoid arthritis is Pulmonary renal syndrome
broad and includes fibrotic lung disease secondary to Malignancy
other collagen vascular disorders, interstitial pul- Idiopathic pulmonary fibrosis
monary fibrosis mediated by infectious agents, med-
ication-induced pulmonary fibrosis, granulomatous
lung disease, chronic hypersensitivity pneumonitis,
asbestos- and other inhaled particle-induced fibro- fection occurs more commonly in those receiving
sis, radiation pneumonitis, recurrent aspiration immunosuppressive agents, it can also occur in the
pneumonitis, amyloidosis, pulmonary renal syn- absence of such therapy.4 – 6 Infection caused by M
drome, malignancy, and idiopathic pulmonary fibro- tuberculosis, cytomegalovirus, or P carinii must be
sis (Table 2). Diagnoses of particular importance in excluded before attributing interstitial changes to
persons with underlying rheumatoid arthritis in- rheumatoid arthritis, because the treatments for
clude atypical infections and interstitial lung dis- these pulmonary conditions differ and the conse-
ease induced by administration of therapeutic quences of misdiagnosis are grave. In particular, the
agents such as methotrexate and gold.3–11 These combination of failure to diagnose infection with M
diseases must be considered and excluded in pa- tuberculosis and institution of corticosteroid therapy
tients with rheumatoid arthritis and interstitial for pulmonary fibrosis can permit the rapid, delete-
lung disease. rious progression of tuberculosis.
Patients with rheumatoid arthritis are predis- The use of certain therapeutic agents for rheuma-
posed to the development of infection. In fact, this toid arthritis can also produce diffuse interstitial
complication is a leading cause of morbidity and changes. Methotrexate, gold compounds, sulfasala-
death of persons with rheumatoid arthritis.2,23 Spe- zine, and D-penicillamine have been associated with
cific infections include bacterial pneumonia caused the development of diffuse interstitial lung dis-
by Streptococcus pneumoniae and septic arthritis ease.7–11 Methotrexate, a folic acid analog commonly
caused by Staphylococcus aureus23. Although pneu- used as an antitumor and immunosuppressive drug,
monia caused by typical bacteria is usually easily is well-known for its potential to cause potentially
distinguishable from pulmonary fibrosis, infections life-threatening pulmonary disease.7 Methotrexate-
by certain microorganisms can closely resemble induced pneumonitis occurs in up to 5% of treated
rheumatoid arthritis-associated interstitial lung persons with rheumatoid arthritis.7,8 Clinical man-
disease; these include Mycobacteria species (espe- ifestations of methotrexate pulmonary toxicity in-
cially M tuberculosis) and cytomegalovirus.3 Pneu- clude dyspnea, nonproductive cough, and fever.
mocystis carinii pneumonia has also been reported These signs and symptoms can be observed in other
in persons with rheumatoid arthritis. Although in- methotrexate-associated lung complications, includ-
84 January 2001 Volume 321 Number 1
Gochuico
Table 3. Potential Pathogenic Factors in Pulmonary Fibrosis coal worker’s pneumoconiosis, glutathione S-trans-
Associated with Rheumatoid Arthritis ferase class with asbestosis).24 –28 Also, an in-
creased frequency of polymorphisms at the HLA-
Genetic factors
HLA-B40 antigen B40 antigen site has been observed in persons with
␣1-protease inhibitor subtype rheumatoid arthritis who have pulmonary fibrosis
Pi M1M2 or obliterative bronchiolitis.14 In patients with rheu-
Pi MZ matoid arthritis, the site that encodes for ␣1-pro-
Pi MS
Alteration in immunologic response tease inhibitor (␣1-Pi) has been reported to be asso-
Aberrant host repair mechanisms ciated with the development of fibrotic lung
disease.12,13
Pi, ␣1-protease inhibitor subtype. The ␣1-Pi gene locus, located at 14q32.1, encodes
multiple allelic variations or subtypes of ␣1-Pi. ␣1-
Pi, a 56-kDa glycoprotein belonging to the serpin
ing hypersensitivity and nonhypersensitivity pneu- family of serine protease inhibitors, is the major
monitis, P carinii infection, and cytomegalovirus protease inhibitor in human serum.29 Although
pneumonia.3,4,7,8 Risk factors identified as predis- ␣1-Pi is capable of interacting with a broad range of
posing patients to methotrexate-induced pulmonary proteases, its primary physiologic target is neutro-
toxicity include advanced age, preexisting intersti- phil elastase. Many ␣1-Pi subtypes, including the
tial disease, and a prior adverse reaction to thera- five Pi M subtypes, are quantitatively and qualita-
peutic agents for rheumatoid arthritis.8 Treatment tively “normal,” as determined by neutrophil elas-
for methotrexate-induced pneumonitis involves dis- tase inhibition. Several uncommon ␣1-Pi allelic vari-
continuation of drug administration, supportive ants, however, are associated with dysfunctional or
care, and possible use of systemic corticosteroids. deficient ␣1-Pi that results from synthesis of cata-
Gold compounds are pharmacologic agents that lytically inactive enzyme or failure of an active pro-
can also be associated with the development of dif- tein to be delivered effectively from hepatocytes to
fuse lung disease. The presence of fever, skin rash, the circulation. Reduced levels of functional ␣1-Pi,
lymphocytosis in BAL fluid, alveolar opacities dis- such as that observed in Pi ZZ variant homozygotes,
tributed along bronchovascular bundles on chest can cause a predisposition to the early development
computed tomography images, and absence of sub- of emphysema, especially in smokers.
cutaneous nodules or digital clubbing are features In contrast to the association between ␣1-Pi phe-
that distinguish gold-induced pulmonary disease notype and development of emphysema, a correla-
from rheumatoid arthritis-associated lung disease.9 tion between ␣1-Pi subtype and the occurrence of
Predisposing factors that identify rheumatoid ar- pulmonary fibrosis in patients with rheumatoid ar-
thritis patients at risk for developing gold-induced thritis is controversial. For example, one study did
lung disease are unknown. Therapy includes cessa- not demonstrate a significant association between Pi
tion of gold therapy, supportive care, and adminis- Z subtype and fibrotic lung disease in patients with
tration of systemic corticosteroids (or steroid-spar- rheumatoid arthritis.30 However, studies involving
ing medications) for severe toxicity.9 limited numbers of patients indicated that some
␣1-Pi heterozygotes (ie, Pi M1M2, Pi MZ, Pi MS)
Pathogenesis were associated with fibrotic lung disease.12,13 Al-
though pathogenic mechanisms remain unknown,
The etiology of pulmonary fibrosis in patients with ␣1-Pi has been reported to reduce fibroblast prolif-
rheumatoid arthritis is unknown. Possible patho- eration by inhibiting transferrin binding to its re-
genic mechanisms include genetic factors, alteration ceptor.31 Because fibroblasts produce collagen and
in immunologic response, and/or aberrant tissue re- other extracellular matrix proteins, this effect may
pair mechanisms (Table 3). contribute to the regulation of fibroblast cell turn-
The potential role of genetic factors in the patho- over and matrix protein synthesis. ␣1-Pi also con-
genesis of pulmonary fibrosis in patients with rheu- tributes to the regulation of immunity through its
matoid arthritis has been investigated.12–14 Inher- modulation of monocyte and macrophage function,
ited host susceptibility factors interacting with lymphocyte proliferation and cytotoxicity.32 Consis-
single or multiple environmental exposures can po- tent with these data, ␣1-Pi administered to ham-
tentially initiate the development of the fibrotic pro- sters treated with bleomycin ameliorated the in-
cess in the lung. In support of this hypothesis, poly- flammation and fibrosis characteristic of this animal
morphisms at several loci have been associated with model of pulmonary fibrosis.33 Thus, it is possible
certain fibrotic lung diseases (eg, immunoglobulin that certain ␣1-Pi subtypes, previously character-
Gm with familial idiopathic pulmonary fibrosis, glu- ized as functionally “normal” (with regard to their
tamate 69 in human leukocyte antigen (HLA)-DP ability to inhibit trypsin and elastase), are not func-
with chronic beryllium disease and hard metal lung tionally normal in their ability to modulate fibro-
disease, HLA-DR8 and tumor necrosis factor-␣ with blast proliferation and immune cell function.
THE AMERICAN JOURNAL OF THE MEDICAL SCIENCES 85
Pulmonary Fibrosis Associated with Rheumatoid Arthritis
Other pathogenetic processes that can potentially Table 4. Radiologic Characteristics of Pulmonary Fibrosis
Associated with Rheumatoid Arthritis
contribute to the development of fibrotic lung dis-
ease in persons with rheumatoid arthritis include Chest roentgenogram
immunologic dysregulation and alteration of tissue Basilar alveolar opacities
repair mechanisms. Histologic findings of intersti- Reticulonodular interstitial densities
tial infiltration by lymphocytes, accumulation of Loss of lung volume
mononuclear cells within the alveolar space, and Honeycombing
High-resolution computed tomography
change in the distribution of T cell subsets suggest Ground-glass opacification
that lymphocytes may be involved in the pathogen- Basal honeycombing
esis of this disorder.1 In addition, alveolar macro- Bronchiectasis
phages have the ability to produce matrix metallo- Coexistent emphysema or pleural disease
Findings suggestive of underlying rheumatoid arthritis
proteinases, cytokines (eg, tumor necrosis factor-␣), Rheumatoid nodules
and growth factors (eg, platelet-derived growth fac- Pleural disease
tor, transforming growth factor-␣ and -, insulin-
like growth factor-I, and basic fibroblast growth
factor) that can promote fibroblast proliferation and
the accumulation of extracellular matrix pro- abnormalities with basilar interstitial changes is
teins.11,34 Furthermore, BAL specimens from per- suggestive of rheumatoid arthritis-associated fi-
sons with rheumatoid arthritis and interstitial lung brotic lung disease.
disease can contain increased numbers of neutro- HRCT of the chest is an important modality that
phils with functional alterations, including elevated should be used to assess patients with fibrotic lung
myeloperoxidase activity and protease produc- disease. A correlation between thin-section HRCT
tion.16 –18 The proteases are believed to contribute to and pathologic fibrosis scores in patients with idio-
the generation of fibrinopeptide A-reactive proteins pathic pulmonary fibrosis has been reported.37 Al-
and collagenase within the lung, consistent with though similar histologic correlations are not avail-
potential effects of neutrophils on both synthesis able for patients with rheumatoid arthritis-related
and degradation of matrix components in this pulmonary fibrosis, HRCT scans of the chest in 44%
disease. of patients with rheumatoid arthritis and suspected
interstitial lung disease demonstrated ground-glass
Clinical Manifestations opacification or basal honeycombing.38 Other com-
monly observed findings include bronchiectasis, em-
The clinical manifestations of pulmonary fibrosis physema, and pleural disease.
associated with rheumatoid arthritis are indistin- Limited data are available regarding the assess-
guishable from those observed in patients with other ment of these patients with radionuclide examina-
chronic fibrotic lung diseases.1,2 The age of onset is tions such as 99m-TcDTPA lung clearance scanning
variable, with a mean reportedly in the fifth to sixth and J001X scintigraphy.39,40 In a small group of
decade of life. Symptoms are insidious, and can patients with a variety of diffuse infiltrative lung
include dyspnea, nonproductive cough, and chest diseases, alveolar permeability as measured by
pain. Physical exam can reveal inspiratory crackles 99m-TcDTPA lung clearance scanning was similar
and digital clubbing. Findings associated with rheu- in both early- and late-stage disease.39 Because the
matoid arthritis such as subcutaneous nodules can utility of 99m-TcDTPA lung clearance scans in pa-
be observed in up to 52%, whereas rheumatoid fac- tients with rheumatoid arthritis and pulmonary fi-
tor is detectable in 70 to 80% of these patients.35 brosis was not specifically assessed, its application
Notably, although the majority of persons with in the management of such patients is unclear.
rheumatoid arthritis present with pulmonary dis-
ease either after or concurrent with the onset of Pulmonary Physiology Characteristics
arthritis, approximately 10% of patients develop
symptomatic lung disease before the development of As in other fibrotic lung disorders, pulmonary
joint disease.1,2 fibrosis in patients with rheumatoid arthritis is
characterized by a restrictive pattern of ventilatory
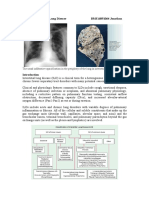
Radiologic Characteristics function and reduced diffusion capacity.35 Such abnor-
malities can be observed in patients with rheumatoid
Roentgenographic features of rheumatoid arthri- arthritis who have normal chest roentgenograms. In a
tis-associated pulmonary fibrosis are variable and cross-sectional study, 10% of nonsmoking patients
include early patchy basilar alveolar opacities, pro- with rheumatoid arthritis had a mild reduction of
gressive reticulonodular interstitial densities with forced vital capacity and 15% had a diffusion capacity
loss of lung volume, and end-stage honeycombing of less than 80% of predicted values; only 5% had
(Table 4).36 Although these findings are nonspecific, detectable interstitial abnormalities on chest films.
the coexistence of rheumatoid nodules or pleural Reduced pulmonary function was more commonly ob-
86 January 2001 Volume 321 Number 1
Gochuico
served in rheumatoid arthritis patients who had evi- Table 5. Therapeutic Considerations for Pulmonary Fibrosis
Associated with Rheumatoid Arthritis
dence of severe disease (ie, circulating rheumatoid
factor, rheumatoid nodules, bony erosions), pulmonary Systemic corticosteroids
symptoms (ie, cough, dyspnea, pedal edema, pleurisy), Azathioprine
or cumulative treatment with methotrexate for Cyclophosphamide
greater than 1 year. Longitudinal evaluation of these Cyclosporine
patients and pathologic confirmation of pulmonary Methotrexate
Penicillamine
fibrosis were not performed. Hydroxychloroquine sulfate
BAL Fluid and Pathology Findings
The cellular constituents in BAL fluid and the
pulmonary pathology in patients with rheumatoid
arthritis and pulmonary fibrosis have been exam- a 5-year survival rate of 39%.19 The prognosis of
ined. The reported BAL fluid findings of patients patients with less severe disease remains unknown.
with rheumatoid arthritis have been generally in-
consistent and nonspecific. This variability is possi- Conclusion
bly related to differences in patients’ disease activ- Pulmonary fibrosis in persons with rheumatoid
ity, quantity of cigarettes smoked, and exposure to arthritis is an uncommon lung disorder that may
certain disease-modifying drugs, including metho- affect more patients than has been recognized thus
trexate, gold compounds, and penicillamine.16,41 Un- far. Atypical infections and drug-induced lung dis-
til further information demonstrating diagnostic or ease are important conditions to exclude in these
prognostic utility of BAL analysis in defined popu- patients. The pathogenesis of the lung fibrosis is
lations with rheumatoid arthritis become available, unknown and may involve genetic host susceptibil-
routine evaluation of BAL cells in all patients with ity, immune cell dysregulation, and/or aberrant tis-
rheumatoid arthritis is not clinically indicated. sue repair mechanisms. The clinical features, heter-
The pathologic features of pulmonary fibrosis in ogeneous disease course, and unfavorable prognosis
patients with rheumatoid arthritis include (in the seem to resemble those that characterize idiopathic
early stages), accumulation of lymphocytes, plasma pulmonary fibrosis. Therapeutic options include em-
cells, and macrophages within arterioles, bronchi- piric trials of systemic corticosteroids and/or steroid-
oles, and interalveolar septa as well as the later sparing agents.
findings of fibrosis, bronchiolar dilation, parenchy-
mal cyst formation with epithelial hyperplasia, and
honeycombing.42,43 Although these features are also References
present in other fibrotic lung diseases, the concom- 1. Roschmann RA, Rothenberg RJ. Pulmonary fibrosis in
itant identification of rheumatoid nodules in lung rheumatoid arthritis: a review of clinical features and ther-
tissue is specific for and diagnostic of rheumatoid apy. Semin Arthritis Rheum 1987;16:174 – 85.
arthritis-associated lung disease. 2. Anaya JM, Espinoza LR, Welsh RA, et al. Pulmonary
involvement in rheumatoid arthritis. Semin Arthritis Rheum
1995;24:242–54.
Therapeutic Considerations and Prognosis 3. Aglas F, Rainer F, Hermann J, et al. Interstitial pneumo-
nia due to cytomegalovirus following low-dose methotrexate
Despite the absence of controlled studies to eval- treatment for rheumatoid arthritis. Arthritis Rheum 1995;38:
uate medical therapy for this disease, several case 291–2.
reports describe clinical responses to systemic corti- 4. Roux N, Flipo RM, Cortet B, et al. Pneumocystis carinii
costeroids, azathioprine, cyclophosphamide, metho- pneumonia in rheumatoid arthritis patients treated with
trexate, and D-penicillamine.1 Administration of methotrexate. A report of two cases. Rev Rhum Engl Ed
1996;63:453– 6.
therapeutic agents for this disease, therefore, is 5. Prekates A, Roussos C, Paniara O, et al. Pneumocystis
based largely on anecdotal experience, and clinical carinii pneumonia in a HIV-seronegative patient with un-
criteria that guide medication selection are unavail- treated rheumatoid arthritis and CD4⫹ T-lymphocytopenia.
able. Drugs that have been used to treat patients Eur Respir J 1997;10:1184 – 6.
with pulmonary fibrosis and rheumatoid arthritis 6. Oien KA, Madhok R, Hunter JA, et al. Pneumocystis
are noted in Table 5. carinii pneumonia in a patient with rheumatoid arthritis, not
Although pulmonary fibrosis is known to be a on immunosuppressive therapy and in the absence of human
leading cause of death in these patients, their prog- immunodeficiency virus infection. Br J Rheumatol 1995;34:
677–9.
nosis is still poorly defined and difficult to assess 7. Cannon GW. Methotrexate pulmonary toxicity. Rheum Dis
because of the heterogeneous nature of the disease.2 Clin North Am 1997;23:917–37.
A small, retrospective analysis of hospitalized per- 8. Ohosone Y, Okano Y, Kameda H, et al. Clinical charac-
sons with rheumatoid arthritis and lung fibrosis teristics of patients with rheumatoid arthritis and methotrex-
reported a median survival of less than 4 years and ate induced pneumonitis. J Rheumatol 1997;24:2299 –303.
THE AMERICAN JOURNAL OF THE MEDICAL SCIENCES 87
Pulmonary Fibrosis Associated with Rheumatoid Arthritis
9. Tomioka H, King TE. Gold-induced pulmonary disease: clinical 27. Rihs HP, Lipps P, May-Taube K, et al. Immunogenetic
features, outcome, and differentiation from rheumatoid lung dis- studies on HLA-DR in German coal miners with and without
ease. Am J Respir Crit Care Med 1997;155:1011–20. coal worker’s pneumoconiosis. Lung 1994;172:347–54.
10. Hamadeh MA, Atkinson J, Smith LJ. Sulfasalazine-in- 28. Smith CM, Kelsey KT, Wiencke JK, et al. Inherited gluta-
duced pulmonary disease. Chest 1992;101:1033–7. thione S-transferase deficiency is a risk factor for pulmonary
11. Scott DL, Bradby GV, Aitman TJ, et al. Relationship of asbestosis. Cancer Epidemiol Biomarkers Prev 1994;3:471–7.
gold and penicillamine therapy to diffuse interstitial lung 29. Norman MR, Mowat AP, Hutchison DCS. Molecular ba-
disease. Ann Rheum Dis 1981;40:136 – 41. sis, clinical consequences and diagnosis of alpha 1-antitryp-
12. Geddes DM, Webley M, Brewerton DA, et al. Alpha1- sin deficiency. Ann Clin Biochem 1997;34:230 – 46.
antitrypsin phenotypes in fibrosing alveolitis and rheumatoid 30. Steers G, McMahon MJ, Grennan DM, et al. Lack of
arthritis. Lancet 1977;ii:1049 –50. association of the alpha 1-antitrypsin PiZ allele with rheu-
13. Michalski JP, McCombs CC, Scopelitis E, et al. Alpha matoid arthritis or with its extra articular complications. Dis
1-antitrypsin phenotypes, including M subtypes, in pulmo- Markers 1992;10:151–7.
nary disease associated with rheumatoid arthritis and sys- 31. Graziadei I, Vogel W, Wiedermann CJ, et al. The acute-
temic sclerosis. Arthritis Rheum 1986;29:586 –91. phase protein alpha 1-antitrypsin inhibits transferrin bind-
14. Charles PJ, Sweatman MC, Markwick JR, et al. HLA- ing and proliferation of human skin fibroblasts. Biochim
B40: a marker for susceptibility to lung disease in rheuma- Biophys Acta 1998;1401:170 – 6.
toid arthritis. Dis Markers 1991;9:97–101. 32. Breit SN, Wakefield D, Robinson JP, et al. The role of
15. Gosset P, Perez T, Lassalle P, et al. Increased TNF-alpha
alpha 1-antitrypsin deficiency in the pathogenesis of immune
secretion by alveolar macrophages from patients with rheu-
disorders. Clin Immunol Immunopathol 1985;35:363– 80.
matoid arthritis. Am Rev Respir Dis 1991;143:593–7. 33. Nagai A, Takizawa T, Kagawa J, et al. Administration of alpha
16. Garcia JGN, James HL, Zinkgraf S, et al. Lower respira-
1-proteinase inhibitor ameliorates bleomycin-induced pulmonary
tory tract abnormalities in rheumatoid interstitial lung dis-
fibrosis in hamsters. Am Rev Respir Dis 1992;145:651–6.
ease. Potential role of neutrophils in lung injury. Am Rev 34. Chan ED, Worthen GS, Augustin A, et al. Inflammation
Respir Dis 1987;136:811–7.
in the pathogenesis of interstitial lung diseases. In: Schwarz
17. Idell S, Garcia JGN, Gonzalez K, et al. Fibrinopeptide A
MI, King Jr TE, editors. Interstitial lung disease, 3rd ed.
reactive peptides and procoagulant activity in bronchoalveo-
Hamilton, Ontario: B.C. Decker; 1998. p. 135– 64.
lar lavage: relationship to rheumatoid interstitial lung dis-
35. Saag KG, Schwartz DA, Hunninghake GW, et al. Rheu-
ease. J Rheumatol 1989;16:592– 8.
matoid arthritis lung disease. Determinants of radiographic and
18. Weiland JE, Garcia JGN, Davis WB, et al. Neutrophil
physiologic abnormalities. Arthritis Rheum 1996;39:1711–9.
collagenase in rheumatoid interstitial lung disease. J Appl
36. Martel W, Abell MR, Mikkelsen WM, et al. Pulmonary
Physiol 1987;62:628 –33.
19. Hakala M. Poor prognosis in patients with rheumatoid ar- and pleural lesions in rheumatoid disease. Radiology 1968;
thritis hospitalized for interstitial lung fibrosis. Chest 1988; 90:641–53.
37. Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section
93:114 – 8.
20. Brannan HM, Good CA, Divertie MB, et al. Pulmonary CT obtained at 10-mm increments versus limited three-level
disease associated with rheumatoid arthritis. JAMA 1964; thin-section CT for idiopathic pulmonary fibrosis: correlation
189:138 – 42. with pathologic scoring. Am J Radiol 1997;169:977– 83.
21. Frank ST, Weg JG, Harkleroad LE, et al. Pulmonary 38. McDonagh J, Greaves M, Wright AR, et al. High resolu-
dysfunction in rheumatoid disease. Chest 1973;63:27–34. tion computed tomography of the lungs in patients with
22. Gabbay E, Lake FR, Cameron D, et al. Interstitial lung rheumatoid arthritis and interstitial lung disease. Br J Rheu-
disease in recent onset rheumatoid arthritis. Am J Respir matol 1994;33:118 –22.
Crit Care Med 1997;156:528 –35. 39. Uh S, Lee SM, Kim HT, et al. The clearance rate of alveolar
23. Soria LM, Escofet DR, Garcia JV, et al. Infectious arthri- epithelium using 99mTc-DTPA in patients with diffuse infil-
tis in patients with rheumatoid arthritis. Ann Rheum Dis trative lung disease. Chest 1994;106:161–5.
1992;51:402–3. 40. Goupille P, Le Pape A, Delarue A, et al. Imaging of
24. Musk AW, Zilko PJ, Manners P, et al. Genetic studies in pulmonary disease in rheumatoid arthritis using J001X-scintig-
familial fibrosing alveolitis. Possible linkage with immuno- raphy: preliminary results. Eur J Nucl Med 1995;22:1411–5.
globulin allotypes (Gm). Chest 1986;89:206 –10. 41. Scherak O, Popp W, Kolarz G, et al. Bronchoalveolar
25. Richeldi L, Saltini C, Sorrentino R. HLA-DPB1 gluta- lavage and lung biopsy in rheumatoid arthritis. In vivo ef-
mate 69: a genetic marker of beryllium disease. Science fects of disease modifying antirheumatic drugs. J Rheumatol
1993;262:242– 4. 1993;20:944 –9.
26. Potolicchio I, Mosconi G, Forni A, et al. Susceptibility to 42. Walker WC, Wright V. Pulmonary lesions and rheumatoid
hard metal lung disease is strongly associated with the pres- arthritis. Medicine 1968;47:501–13.
ence of glutamate 69 in HLA-DP chain. Eur J Immunol 43. Yousem SA, Colby TV, Carrington CB. Lung biopsy in
1997;27:2741–3. rheumatoid arthritis. Am Rev Respir Dis 1985;131:770 –7.
88 January 2001 Volume 321 Number 1
You might also like
- Pulmonary Manifestations of Primary Immunodeficiency DiseasesFrom EverandPulmonary Manifestations of Primary Immunodeficiency DiseasesSeyed Alireza MahdavianiNo ratings yet
- Lung Disease Associated with Rheumatoid ArthritisFrom EverandLung Disease Associated with Rheumatoid ArthritisTakahisa GonoNo ratings yet
- Gupta 2015Document13 pagesGupta 2015Juan Diego CamposNo ratings yet
- Maed 06 224Document6 pagesMaed 06 224rizki romadaniNo ratings yet
- ARDS Causes Diffuse Alveolar Damage in The LungDocument5 pagesARDS Causes Diffuse Alveolar Damage in The LungArfyanda ZakariaNo ratings yet
- S2173574318300728Document7 pagesS2173574318300728Sergiu AlupoaeNo ratings yet
- Infectious Complications in Patients With Lung Cancer: K.S. Akinosoglou, K. Karkoulias, M. MarangosDocument11 pagesInfectious Complications in Patients With Lung Cancer: K.S. Akinosoglou, K. Karkoulias, M. Marangosabbhyasa5206No ratings yet
- Treatment of Slowly Growing Mycobacteria: Julie V. Philley,, David E. GriffithDocument12 pagesTreatment of Slowly Growing Mycobacteria: Julie V. Philley,, David E. Griffiththanhnhat5521No ratings yet
- Description of DiseaseDocument10 pagesDescription of DiseaseRajaNo ratings yet
- Rheumatoid Lung DisDocument28 pagesRheumatoid Lung DisariceghaNo ratings yet
- Pulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (Ards) : A Case Report and Review of LiteratureDocument5 pagesPulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (Ards) : A Case Report and Review of Literatureamelya asryNo ratings yet
- Tuberculosis and Lung Damage: From Epidemiology To PathophysiologyDocument20 pagesTuberculosis and Lung Damage: From Epidemiology To PathophysiologyDyan TonyNo ratings yet
- Necrotizing Pneumonia (Aetiology, Clinical Features and Management)Document8 pagesNecrotizing Pneumonia (Aetiology, Clinical Features and Management)pachomdNo ratings yet
- Pulmonary Infiltrates in The Non-HIV-Infected Immunocompromised PatientDocument12 pagesPulmonary Infiltrates in The Non-HIV-Infected Immunocompromised PatientAri KuncoroNo ratings yet
- ERS MONOGRAPH - PULMONARY MANIFESTATIONS OF SYSTEMIC DISEASES/ Artritis ReumatoideaDocument24 pagesERS MONOGRAPH - PULMONARY MANIFESTATIONS OF SYSTEMIC DISEASES/ Artritis ReumatoidearocioanderssonNo ratings yet
- SDL 18 Interstitial Lung Disease BMS16091064Document8 pagesSDL 18 Interstitial Lung Disease BMS16091064Jonathan YeohNo ratings yet
- Viral Pneumoni Dari 18Document13 pagesViral Pneumoni Dari 18Yoyada SitorusNo ratings yet
- Gharib2001 Radiologi Pneumonia-DikonversiDocument31 pagesGharib2001 Radiologi Pneumonia-Dikonversitsamara24_No ratings yet
- Chapter 10 Interstitial Lung DiseaseDocument10 pagesChapter 10 Interstitial Lung DiseaseAleksandar Tasic100% (1)
- Interstitial Pneumonia Seminar PaperDocument9 pagesInterstitial Pneumonia Seminar PaperLamy SNo ratings yet
- Non-Tuberculous Mycobacterial Pulmonary Infections: ReviewDocument12 pagesNon-Tuberculous Mycobacterial Pulmonary Infections: Reviewmgoez077No ratings yet
- Rheumatic Fever & RHDDocument13 pagesRheumatic Fever & RHDValerrie NgenoNo ratings yet
- DMTCDocument26 pagesDMTCfabricio duarteNo ratings yet
- Diagnosis and Treatment of Diffuse Interstitial Lung DiseasesDocument21 pagesDiagnosis and Treatment of Diffuse Interstitial Lung Diseaseswajeeh rehmanNo ratings yet
- Armando Hasudungan: AuthorsDocument1 pageArmando Hasudungan: AuthorsShubham TarapureNo ratings yet
- Study Guide 2Document16 pagesStudy Guide 2cNo ratings yet
- Neumonia NecrotizanteDocument8 pagesNeumonia Necrotizantearturo garciaNo ratings yet
- Viewpoint: Dennis Mcgonagle, James S O'Donnell, Kassem Sharif, Paul Emery, Charles BridgewoodDocument9 pagesViewpoint: Dennis Mcgonagle, James S O'Donnell, Kassem Sharif, Paul Emery, Charles BridgewoodMonicafajrinNo ratings yet
- Bronchiectasis Outcome and Risk Factors of Mortality in Port SudanDocument5 pagesBronchiectasis Outcome and Risk Factors of Mortality in Port Sudanajmrr editorNo ratings yet
- 127+Layout+JR+8 (2) +DR +Budi+Yanti+ (P 87-93) +Document7 pages127+Layout+JR+8 (2) +DR +Budi+Yanti+ (P 87-93) +Julian HutabaratNo ratings yet
- 1 s2.0 S0140673622010522 MainDocument18 pages1 s2.0 S0140673622010522 MainsilviaNo ratings yet
- Macintyre 2008Document6 pagesMacintyre 2008adek07No ratings yet
- Artigo Doenças Pulmonares Relacionadas Ao TabagismoDocument15 pagesArtigo Doenças Pulmonares Relacionadas Ao TabagismoansidnaisssNo ratings yet
- The Kursk State Medical University: Lecture For Self-Training of 6-th Medical Course English-Speaking StudentsDocument15 pagesThe Kursk State Medical University: Lecture For Self-Training of 6-th Medical Course English-Speaking StudentsDaniel FunkNo ratings yet
- Aen Kelompok 1Document10 pagesAen Kelompok 1mahkda anjaniNo ratings yet
- COPD ImmunopathologyDocument19 pagesCOPD ImmunopathologyLevente BalázsNo ratings yet
- Pi Is 0749070421000397Document14 pagesPi Is 0749070421000397Vlady78No ratings yet
- Evidence-Based Clinical Practice Guidelines Chronic Cough Due To Bronchiectasis: ACCPDocument12 pagesEvidence-Based Clinical Practice Guidelines Chronic Cough Due To Bronchiectasis: ACCPAlizaPinkyNo ratings yet
- SiñomulmonDocument14 pagesSiñomulmonDra Daphne Rivero GallegosNo ratings yet
- PR JE Mikro Jenjang 1 Frany FinalDocument5 pagesPR JE Mikro Jenjang 1 Frany FinalFrany CharismaNo ratings yet
- Pneumonia em Pac Com Cancer PDFDocument15 pagesPneumonia em Pac Com Cancer PDFleca_medica20144849No ratings yet
- 1 s2.0 S0140673622010522 MainDocument18 pages1 s2.0 S0140673622010522 MainMirella Rugel SocolaNo ratings yet
- Pneumonia ComunitariaDocument10 pagesPneumonia ComunitariaMaulida HalimahNo ratings yet
- Chest: Recent Advances in Chest MedicineDocument9 pagesChest: Recent Advances in Chest MedicinesmansakobiNo ratings yet
- Altenburg J Review Article Neth J Med 2015Document8 pagesAltenburg J Review Article Neth J Med 2015manalNo ratings yet
- The Pathophysiology and Dangers of Silent Hypoxemia in COVID-19 Lung InjuryDocument24 pagesThe Pathophysiology and Dangers of Silent Hypoxemia in COVID-19 Lung Injurypulmo unandNo ratings yet
- Printed MaterialsDocument10 pagesPrinted Materialsjesiegutay21No ratings yet
- The Tree in Bud SignDocument2 pagesThe Tree in Bud SignMunish GuleriaNo ratings yet
- Jurnal Bahasa InggrisDocument12 pagesJurnal Bahasa InggrisVanessa Angelica SitepuNo ratings yet
- Bio Markers in ArdsDocument20 pagesBio Markers in ArdsDr. Anusha SambandamNo ratings yet
- Fpi Nejm Clincal Practice 2018Document13 pagesFpi Nejm Clincal Practice 2018cjonderedsonNo ratings yet
- Ijccm 25 S150Document5 pagesIjccm 25 S150Carlos MejiaNo ratings yet
- Lepto Spiros IsDocument11 pagesLepto Spiros IsagathageraldyneNo ratings yet
- Interstitial Lung DiseaseDocument77 pagesInterstitial Lung DiseaseMahaveer S ShekhawatNo ratings yet
- Clinical Presentation and Diagnosis of Pneumocystis Pulmonary Infection in HIV-infected PatientsDocument23 pagesClinical Presentation and Diagnosis of Pneumocystis Pulmonary Infection in HIV-infected PatientsAndréia GomesNo ratings yet
- J of Thrombosis Haemost - 2021 - Khismatullin - Pathology of Lung Specific Thrombosis and Inflammation in COVID 19Document11 pagesJ of Thrombosis Haemost - 2021 - Khismatullin - Pathology of Lung Specific Thrombosis and Inflammation in COVID 19Iulia LupascuNo ratings yet
- COP - Cryptogenic Organizing Pneumonia - StatPearls - NCBI BookshelfDocument7 pagesCOP - Cryptogenic Organizing Pneumonia - StatPearls - NCBI Bookshelfrbatjun576No ratings yet
- Respiratory Medicine Case ReportsDocument8 pagesRespiratory Medicine Case ReportsdiahNo ratings yet
- Idiopathic Pulmonary FibrosisDocument13 pagesIdiopathic Pulmonary FibrosisRaul David Maldonado LearyNo ratings yet
- Common Causes of Paediatric Alopecia: EpidemiologyDocument5 pagesCommon Causes of Paediatric Alopecia: EpidemiologyLiya SuwarniNo ratings yet
- 5 Lecture Preparation Histological SpecimensDocument37 pages5 Lecture Preparation Histological SpecimensLiya SuwarniNo ratings yet
- Global Osteitis Scoring Scale and Chronic Rhinosinusitis: A Marker of Revision SurgeryDocument7 pagesGlobal Osteitis Scoring Scale and Chronic Rhinosinusitis: A Marker of Revision SurgeryLiya SuwarniNo ratings yet
- Community Medicine Module Book PDFDocument37 pagesCommunity Medicine Module Book PDFLiya SuwarniNo ratings yet
- JR Congenital Moleculare PAHDocument19 pagesJR Congenital Moleculare PAHLiya SuwarniNo ratings yet
- Can An Atomic Force Microscope Sequence DNA Using A Nanopore?Document8 pagesCan An Atomic Force Microscope Sequence DNA Using A Nanopore?Liya SuwarniNo ratings yet
- Daftar Pustaka: Fakultas Kedokteran Universitas AndalasDocument8 pagesDaftar Pustaka: Fakultas Kedokteran Universitas AndalasLiya SuwarniNo ratings yet
- Forty-Eighth National Meeting of The Child Neurology SocietyDocument177 pagesForty-Eighth National Meeting of The Child Neurology SocietyLiya SuwarniNo ratings yet
- Bowl-And-Snail Technique For Soft Cataract: Ahmed Gomaa, FRCS, PHD, Christopher Liu, FrcophthDocument3 pagesBowl-And-Snail Technique For Soft Cataract: Ahmed Gomaa, FRCS, PHD, Christopher Liu, FrcophthLiya SuwarniNo ratings yet
- Coronavirus (COVID-19) Infection in Pregnancy: Information For Healthcare ProfessionalsDocument61 pagesCoronavirus (COVID-19) Infection in Pregnancy: Information For Healthcare ProfessionalsLiya SuwarniNo ratings yet
- Diatoms Fundamentals and ApplicationsDocument9 pagesDiatoms Fundamentals and ApplicationsLiya Suwarni0% (1)
- Second Medical Use CountriesDocument5 pagesSecond Medical Use CountriesAnonymous AWcEiTj5u0No ratings yet
- Manual of Bone Densitometry Measurements - An Aid To The Interpretation of Bone Densitometry Measurements in A Clinical Setting PDFDocument229 pagesManual of Bone Densitometry Measurements - An Aid To The Interpretation of Bone Densitometry Measurements in A Clinical Setting PDFsesjrsNo ratings yet
- CeftriaxoneDocument6 pagesCeftriaxonealfredolealcorvoNo ratings yet
- 4 5774036780135220376 PDFDocument102 pages4 5774036780135220376 PDFAhmed Fikry100% (2)
- Probable Mode of Action of Sanjivani Vati - A Critical ReviewDocument13 pagesProbable Mode of Action of Sanjivani Vati - A Critical ReviewAlna TechnicalNo ratings yet
- By Dr. Kamran Javed Naquvi,: Unit II Pharmacognosy (DP-103)Document31 pagesBy Dr. Kamran Javed Naquvi,: Unit II Pharmacognosy (DP-103)Bashar AhmedNo ratings yet
- Efficacy of Caffeine in Promoting Hair Growth by Enhancing Intracellular Activity of Hair FolliclesDocument8 pagesEfficacy of Caffeine in Promoting Hair Growth by Enhancing Intracellular Activity of Hair FolliclesElsayed Refaat Aly MareyNo ratings yet
- Toltrazuril-Plumbs Veterinary HandbookDocument2 pagesToltrazuril-Plumbs Veterinary Handbookmili349No ratings yet
- Daftar Obat Lasa 2022 (II)Document2 pagesDaftar Obat Lasa 2022 (II)Ekta Suci WahyuniNo ratings yet
- Dental Disease in Pet Rabbits 3 Jaw AbscessesDocument11 pagesDental Disease in Pet Rabbits 3 Jaw AbscessesBrvo CruzNo ratings yet
- Images: All Videos Books News Maps FlightsDocument1 pageImages: All Videos Books News Maps FlightsChan ParinaNo ratings yet
- Study-Guide-2-Management-of-Patients-with-Cardiovacular-Copy For StudentsDocument17 pagesStudy-Guide-2-Management-of-Patients-with-Cardiovacular-Copy For StudentsDan Dan ManaoisNo ratings yet
- Shrinivas Product ListDocument32 pagesShrinivas Product ListAniket BhosaleNo ratings yet
- 12 7Document424 pages12 7حسين عبيدينNo ratings yet
- Antibiotics-An Investigatory ProjectDocument20 pagesAntibiotics-An Investigatory ProjectAdiJaijan100% (2)
- A Course of Home Study For Pharmacists (1896)Document620 pagesA Course of Home Study For Pharmacists (1896)dluker2pi1256No ratings yet
- #04 WN Ms Sheila Cartwright Case Notes Unstable Diabetes MellitusDocument2 pages#04 WN Ms Sheila Cartwright Case Notes Unstable Diabetes MellitusNurse OetNo ratings yet
- Pengaruh Pemberian Simetidin Terhadap Profil Farmakokinetika Parasetamol Dengan Metode High Performance Liquid TAHUN 2020Document8 pagesPengaruh Pemberian Simetidin Terhadap Profil Farmakokinetika Parasetamol Dengan Metode High Performance Liquid TAHUN 2020hanifah nofilaNo ratings yet
- Timmen L. Cermak - From Bud To Brain - A Psychiatrist's View of Marijuana (2020, Cambridge University Press) - Libgen - LiDocument246 pagesTimmen L. Cermak - From Bud To Brain - A Psychiatrist's View of Marijuana (2020, Cambridge University Press) - Libgen - LiChadaratch UdomswangchokeNo ratings yet
- Acute and Sub-Acute Oral Toxicity Lagerstroemia Speciosa in Sprague-Dawley RatsDocument7 pagesAcute and Sub-Acute Oral Toxicity Lagerstroemia Speciosa in Sprague-Dawley RatssovalaxNo ratings yet
- Study of Effect of Pilocarpine On Rabbit EyeDocument3 pagesStudy of Effect of Pilocarpine On Rabbit EyeSajjad AliNo ratings yet
- Inui 2014 - A Breast Cancer Patient Treated With GcMAFDocument5 pagesInui 2014 - A Breast Cancer Patient Treated With GcMAFDavid MartínezNo ratings yet
- Quiz NPWDocument5 pagesQuiz NPWChanchal BadhaiNo ratings yet
- Cerebral Protection: BY DR - Suneesh Thilak Registrar Anaesthesia Sut Hospital, PattomDocument30 pagesCerebral Protection: BY DR - Suneesh Thilak Registrar Anaesthesia Sut Hospital, PattomsuneeshNo ratings yet
- Theories and Mechanisms of Dissolution Testing: D.Narender M.PH Arma Cy1 Seme SterDocument32 pagesTheories and Mechanisms of Dissolution Testing: D.Narender M.PH Arma Cy1 Seme SterANo ratings yet
- Full Download Book Browns Atlas of Regional Anesthesia 2 PDFDocument41 pagesFull Download Book Browns Atlas of Regional Anesthesia 2 PDFgeorge.pittman905100% (16)
- Consideration of Steroids For Endodontic PainDocument11 pagesConsideration of Steroids For Endodontic PainArturo Trejo VeraNo ratings yet
- Adhesive ArachnoiditisDocument8 pagesAdhesive ArachnoiditisStaporn KasemsripitakNo ratings yet
- Vasopressin PDFDocument4 pagesVasopressin PDFAnonymous hF5zAdvwCCNo ratings yet
- Osteoporosis, Osteomyellitis, Etc Up To EndDocument39 pagesOsteoporosis, Osteomyellitis, Etc Up To EndJordz PlaciNo ratings yet