Professional Documents
Culture Documents
Measure pHPZC using pH drift
Uploaded by
paola aldanaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Measure pHPZC using pH drift
Uploaded by
paola aldanaCopyright:
Available Formats
How to measure the pHPZC using the pH drift method
Km Ganesh - Sri Sathya Sai Institute of Higher Learning
Anantapur, Andhra Pradesh, India
2015
• Make 0.01 M solution of NaCl.
• Take 50 ml of this solution and bubble it with Nitrogen gas to expel the dissolved CO2
for few minutes in a jacketed titration vessel at room temperature (25 degree Celsius) till
you get a stable pH reading. If nitrogen gas bubbling is not feasible in your lab then some
researchers boil the water to get rid of dissolved CO2 and they keep it in an air tight
condition. Basically, the initial pH should be stable, which varies under exposure to open
air because of dissolution of CO2 which leads to reduction of pH.
• Then adjust the pH of the NaCl solution to pH 2 (use HCl or NaOH solutions for pH
adjustment) [Add few drops at a time and see the trend of change in pH. See that you
don’t end up adding too much HCl or NaOH solution fluctuating up and down around 2
in the effort of getting exact pH 2! Even if it is 1.8 to 1.9 or 2.1 or 2.2, stop there. Note
the initial pH reading once it is stabilized under a close jacketed air tight condition. Add
150 mg of material into the flask containing 50 ml of NaCl solution already adjusted to
pH 2 and keep it for shaking for 24 h in an air tight condition at 200 rpm and 25 degree
Celsius.
• Repeat the same process to get Initial pH of 4, 6, 8, 10 and 12, and then add 150 mg of
adsorbent material after the pH adjustment, and keep each of them for shaking at 200
rpm for 24 hrs.
Note: a. Add the material into the solution after the initial pH is adjusted to 2,4,6,8,10,12.
b. You can scale down the material by half, say 75 mg/25 ml in case you are short
of material and, also take the reading for pH range of 2 to 10 instead of pH range of
2 to 12.
• After 24 h of shaking, filter and measure the final pH for each set.
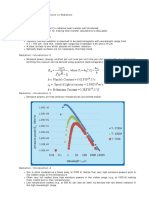
• Plot the final pH (y axis) vs Initial pH (x axis) as shown in figure 1.
Figure 1. pH final vs pH initial.
• Draw a line where initial pH is equal to final pH as shown by the black line in the figure
1.
• As you can see in the figure 1, initial pH 12 led to final pH 11.2, implying that the
adsorbent material is of positive surface charge or acidic [Which attracts and adsorbs
the hydroxide ions from the solution (from the hydronium-hydroxide equilibrium in any
aqueous solution) and reduces its pH from 12 to 11.2]
• Same happens to the set with initial pH 10 that comes down to around pH 8. Next, we
check the final pH for 6. It is clearly on the region (above the middle diagonal line) where
the final pH is above 7 where the initial pH was 6. Implying? The adsorbent surface has
become negative in charge (Basic character) and is removing the positively charged
hydronium ions from the aqueous media. Thus, the final pH is more than initial pH as
reflected in the graph.
Remember: More pH implies less H3O+ ions in the system and lesser the pH implies more
H3O+ ions in the solution. Just to be specific here: solution with pH 2 has 10 times more
hydronium ions in the solution compared to pH 3 or 100 times more than pH 4 or 1000 time
more than pH 5 and so on. It is logarithmic scale, isn’t it?
• Now we have to find the point of transition of the adsorbent material where the surface
charge becomes negative (green zone in the diagram) from the positive (red zone), i.e.
the point where it turns basic from acidic (at the transition the charge will be zero). We
connect the point for pH 8 and pH 6 and see that the line cuts the black diagonal line at
pH 7.4 in this particular case. At this pH the surface charge of the material is zero. Here
is the point where both the initial and the final pH is same after the 24 h shaking.
You might also like
- Determination of Ka of Unknown AcidDocument23 pagesDetermination of Ka of Unknown AcidShasha0% (1)
- Equilibrium Constant For Hydrolysis Lab6finalDocument9 pagesEquilibrium Constant For Hydrolysis Lab6finalapi-534386927No ratings yet
- NMR Kinetics: Study of A Reversible Hydrolysis ReactionDocument8 pagesNMR Kinetics: Study of A Reversible Hydrolysis ReactionOldbooklover100% (2)
- Half Titration Lab ReportDocument6 pagesHalf Titration Lab Reportapi-20078641867% (3)
- Abstract (Lab 2) Ionization ConstantDocument12 pagesAbstract (Lab 2) Ionization Constantmirdza94No ratings yet
- Titration of A Diprotic Acid: Identifying An Unknown by Dan HolmquistDocument8 pagesTitration of A Diprotic Acid: Identifying An Unknown by Dan HolmquistPaul Schumann0% (1)
- Instrumental Analytical Methods Experiment 8 - Conductometric Titration of Sulfuric Acid With Sodium BaseDocument3 pagesInstrumental Analytical Methods Experiment 8 - Conductometric Titration of Sulfuric Acid With Sodium Baseapi-235187189No ratings yet
- Homework 1: HaktarfoneDocument3 pagesHomework 1: HaktarfonePaige D.No ratings yet
- pH Electrode Titration Curve AnalysisDocument14 pagespH Electrode Titration Curve AnalysisMina VoNo ratings yet
- EDTA Complex Formation and Metal Ion TitrationsDocument6 pagesEDTA Complex Formation and Metal Ion TitrationsMark ReyesNo ratings yet
- Bioprocess BasicsDocument365 pagesBioprocess BasicssaveenaNo ratings yet
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- FLA 05 Latent Heat - ELECCIONDocument2 pagesFLA 05 Latent Heat - ELECCIONSheraldene Chavez Eleccion100% (1)
- Standardization of Acid and Base Solutions PDFDocument3 pagesStandardization of Acid and Base Solutions PDFKassim100% (1)
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDocument5 pagesAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- The Titration of Clay MineralsDocument10 pagesThe Titration of Clay MineralsroyamohamadyNo ratings yet
- PH Meter and Conductivity MeterDocument4 pagesPH Meter and Conductivity MeterjeysonmacaraigNo ratings yet
- Preparation and Testing of Acetic Acid-Sodium Acetate Buffer at pH 5Document4 pagesPreparation and Testing of Acetic Acid-Sodium Acetate Buffer at pH 5Laila FaeizahNo ratings yet
- Acetic Acid Dissociation Constant S11Document7 pagesAcetic Acid Dissociation Constant S11Ayesha ShahidNo ratings yet
- Science - Temperature of Sodium Thiosulphate and Rate of ReactionDocument4 pagesScience - Temperature of Sodium Thiosulphate and Rate of ReactionSmartPurdyNo ratings yet
- Acid Neutralizing Capacity of An AntacidDocument4 pagesAcid Neutralizing Capacity of An AntacidibdpNo ratings yet
- Acid Base Titration ExperimentDocument2 pagesAcid Base Titration ExperimentDark_KiroNo ratings yet
- Determination of Biological Oxygen Demand (BOD) in Waste Water - Pharmaceutical GuidelinesDocument3 pagesDetermination of Biological Oxygen Demand (BOD) in Waste Water - Pharmaceutical GuidelinesIrfan SalimNo ratings yet
- Experiment - Determination of Angle of Rotation of A Sugar Solution. Organic Chemistry II Lab ManualDocument5 pagesExperiment - Determination of Angle of Rotation of A Sugar Solution. Organic Chemistry II Lab Manualusman1200550% (1)
- Lab Report 11Document3 pagesLab Report 11PaulNo ratings yet
- Class 11 Chemistry Notes 2023-24 8. Redox ReactionsDocument40 pagesClass 11 Chemistry Notes 2023-24 8. Redox ReactionsAyushi Shah100% (1)
- Experiment No 18Document4 pagesExperiment No 18Suvrasoumya Mohanty100% (2)
- Expt.1 BiochemDocument4 pagesExpt.1 BiochemMc de RamosNo ratings yet
- Acid Base TitrationDocument57 pagesAcid Base TitrationRichard Obinna100% (1)
- CHM170L Exp2 DensityDocument6 pagesCHM170L Exp2 DensityKaiser SaltoNo ratings yet
- HACH Silica in Water-Silico Molybdate Method No. 8185-DOC316.53.01133 PDFDocument6 pagesHACH Silica in Water-Silico Molybdate Method No. 8185-DOC316.53.01133 PDFBalas43No ratings yet
- University of Zimbabwe: 2017 Nov/Dec ExaminationsDocument2 pagesUniversity of Zimbabwe: 2017 Nov/Dec Examinationsmabuto tichaona cNo ratings yet
- Practical Analytical 1 ,,chemistryDocument45 pagesPractical Analytical 1 ,,chemistryFadlin AdimNo ratings yet
- SCYA2101 Engineering Chemistry Lab Manual Final Copy For WebsiteDocument41 pagesSCYA2101 Engineering Chemistry Lab Manual Final Copy For WebsiteSivaSaiNo ratings yet
- Complex Ion Equilibria ExplainedDocument21 pagesComplex Ion Equilibria ExplainedTaulant SheqerxhiuNo ratings yet
- Determination of Na and K by flame photometryDocument2 pagesDetermination of Na and K by flame photometryDebleena ChakrabortyNo ratings yet
- Experiment 1 Potentiometric Titration: Joan Marie Ilagan Joanne Sasondoncillo Ma. Kezia TayagDocument11 pagesExperiment 1 Potentiometric Titration: Joan Marie Ilagan Joanne Sasondoncillo Ma. Kezia Tayagjoai_11No ratings yet
- Conductometric Titrations: Hasan Qayyum Chohan, Reg. No. 2009-CH-204 University of Engineering & Technology Lahore (KSK)Document9 pagesConductometric Titrations: Hasan Qayyum Chohan, Reg. No. 2009-CH-204 University of Engineering & Technology Lahore (KSK)cutetamtam101No ratings yet
- Phase Diagram of Three-Component Liquid SystemDocument11 pagesPhase Diagram of Three-Component Liquid SystemVanessa Denise Aguilar100% (2)
- VinegarDocument14 pagesVinegarLynn HeimatotoNo ratings yet
- Analytical Chemistry Notes IiDocument9 pagesAnalytical Chemistry Notes IiJabez MatigaNo ratings yet
- 5.back & Blank TitrationDocument5 pages5.back & Blank Titration175-44-Faraz HussainNo ratings yet
- 5 PH MeterDocument8 pages5 PH MeterManelleTulodNo ratings yet
- Analytical ChemistryDocument95 pagesAnalytical ChemistryHugo WNo ratings yet
- Cement AnalysisDocument4 pagesCement AnalysisDaryl McCollNo ratings yet
- Anal Chem 3 - Test 1-2016Document4 pagesAnal Chem 3 - Test 1-2016Buhle BuhleNo ratings yet
- CRE Lab ManualDocument19 pagesCRE Lab ManualMayursinh Solanki100% (1)
- Experiment 1 LabDocument9 pagesExperiment 1 LabPatrickNo ratings yet
- Chem Project AntacidDocument20 pagesChem Project Antacidapi-371218886% (7)
- AP Chemistry - Acid Dissociation Constant Ka LabDocument4 pagesAP Chemistry - Acid Dissociation Constant Ka LabJonathan Chen83% (6)
- Glass Electrode. Construction: M Silver Chloride Electrode Calomel ElectrodeDocument4 pagesGlass Electrode. Construction: M Silver Chloride Electrode Calomel ElectrodeYogesh RanwaNo ratings yet
- Acid Neutralizing Capacity of An Antacid: BackgroundDocument5 pagesAcid Neutralizing Capacity of An Antacid: BackgroundNabilah HarisNo ratings yet
- Spectrophotometric Determination of The Acid Dissociation Constant of Methyl RedDocument3 pagesSpectrophotometric Determination of The Acid Dissociation Constant of Methyl Red7063673nasNo ratings yet
- Titration Curves & Equivalence Point (Article) - Khan AcademyDocument21 pagesTitration Curves & Equivalence Point (Article) - Khan AcademyFaiz KhanNo ratings yet
- Kinetics of Ester Hydrolysis NewDocument3 pagesKinetics of Ester Hydrolysis Newbits_who_am_iNo ratings yet
- Synthesis of New Schiff Base From Natural Products For Remediation of Water Pollution With Heavy Metals in Industrial AreasDocument10 pagesSynthesis of New Schiff Base From Natural Products For Remediation of Water Pollution With Heavy Metals in Industrial AreasChern YuanNo ratings yet
- Volumetric Analysis All Till This DateDocument28 pagesVolumetric Analysis All Till This DateSolomonNo ratings yet
- SaponificationDocument35 pagesSaponificationsemanasemana80% (5)
- Buffer and Buffer Capacity Activity ExplainedDocument2 pagesBuffer and Buffer Capacity Activity ExplainedValenzuela Allene GraceNo ratings yet
- PH DRIFTDocument2 pagesPH DRIFTUtsav DalalNo ratings yet
- Measuring pH Using Acids, Bases, and BuffersDocument10 pagesMeasuring pH Using Acids, Bases, and BuffersChing Wai Yong67% (3)
- Tropical Design by Froilan HongDocument3 pagesTropical Design by Froilan HongLawrence TingNo ratings yet
- Experiment 3 (Sublimation and Melting Point Determination)Document13 pagesExperiment 3 (Sublimation and Melting Point Determination)Cheng BauzonNo ratings yet
- NSEJS Camp Equilibrium AssignmentDocument5 pagesNSEJS Camp Equilibrium Assignmentaryan aggarwalNo ratings yet
- Iso 7726-1998Document8 pagesIso 7726-1998felo345No ratings yet
- Ionic Equilibrium Important Problems For JEE Main and JEE AdvancedDocument58 pagesIonic Equilibrium Important Problems For JEE Main and JEE Advancedeswar manchaalaNo ratings yet
- 18.1 and 18.2 HL Part QuestionsDocument7 pages18.1 and 18.2 HL Part QuestionsAlshaimaa SolimanNo ratings yet
- CHEM 18 PROBLEM SET CHEMICAL THERMODYNAMICS AND EQUILIBRIUMDocument4 pagesCHEM 18 PROBLEM SET CHEMICAL THERMODYNAMICS AND EQUILIBRIUMDaniel Jann CotiaNo ratings yet
- SL Paper 1: Incorrect For A 0.10 Mol DM HCOOH Solution?Document11 pagesSL Paper 1: Incorrect For A 0.10 Mol DM HCOOH Solution?Sai SuhasNo ratings yet
- Temperature Measurement TechniquesDocument11 pagesTemperature Measurement Techniquessyafc99No ratings yet
- Heat Transfer EquationsDocument163 pagesHeat Transfer Equationsdragon forceNo ratings yet
- Chen19111005 M JaishDocument20 pagesChen19111005 M JaishUsama Jahangir KhanNo ratings yet
- HMT - V - Mass TransferDocument15 pagesHMT - V - Mass TransferKummitha Obula ReddyNo ratings yet
- Heat ExchangersBasics Design ApplicationsDocument598 pagesHeat ExchangersBasics Design ApplicationsPujara Manish100% (3)
- CH11 2Document20 pagesCH11 2Usaid KhanNo ratings yet
- Refrigeration and Air Conditioning (2161908) : A Laboratory Manual ForDocument62 pagesRefrigeration and Air Conditioning (2161908) : A Laboratory Manual ForAbhishek RaiNo ratings yet
- Design Development and Performance Evaluation of Solar Dryer For Drying of Tomato and Onion Slices PDFDocument98 pagesDesign Development and Performance Evaluation of Solar Dryer For Drying of Tomato and Onion Slices PDFErin Walker100% (4)
- 50 Lab Calorimetry of A Cold Pack GolestanehDocument10 pages50 Lab Calorimetry of A Cold Pack GolestanehnanaNo ratings yet
- Edexcel Module Heat Transfer and Combustion - H2 Outcome 2 - Tutorial 1Document10 pagesEdexcel Module Heat Transfer and Combustion - H2 Outcome 2 - Tutorial 1konticvNo ratings yet
- Module 3: Radiation Lecture 26: Introduction To Radiation Objectives in This ClassDocument8 pagesModule 3: Radiation Lecture 26: Introduction To Radiation Objectives in This ClassUr MahiNo ratings yet
- 2 MidtermsDocument23 pages2 Midtermstorreb8396No ratings yet
- MEC 321 Heat Transfer: Dr. Mohamed Salem ElmnefiDocument15 pagesMEC 321 Heat Transfer: Dr. Mohamed Salem ElmnefiAnonim KopyacıNo ratings yet
- Lab Manual - Practical 5 - Determination of Buffer CapacityDocument3 pagesLab Manual - Practical 5 - Determination of Buffer Capacitysandi fernando100% (1)
- Fabrication of An Evaporative Condenser For Enhancement of C.O.P of An Ac UnitDocument71 pagesFabrication of An Evaporative Condenser For Enhancement of C.O.P of An Ac UnitNaresh DamaNo ratings yet
- Hvac Engineer Interview 70 Question AnswersDocument12 pagesHvac Engineer Interview 70 Question AnswersRobin DittoNo ratings yet
- Acid Base PracticeDocument5 pagesAcid Base PracticeKarn VimolVattanasarnNo ratings yet
- Passive CoolingDocument79 pagesPassive CoolingBirimumaso DavidNo ratings yet
- Kettle Reboiler Design and Heat Transfer AnalysisDocument4 pagesKettle Reboiler Design and Heat Transfer AnalysisSmit patelNo ratings yet
- Surface Observation FormDocument4 pagesSurface Observation Formhadprince2023No ratings yet
- Polimorfismos de Benzocaina PDFDocument9 pagesPolimorfismos de Benzocaina PDFDiegoAndrésYiZapataNo ratings yet