Professional Documents
Culture Documents
Po ZD ACq D9 e H8 RCH O1 BHH Ik 4 N Cbxpat 123531
Uploaded by
zhang chao0 ratings0% found this document useful (0 votes)
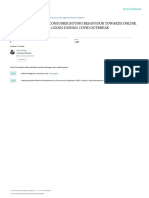
13 views1 pageLuis Colon-Rivera from Hacienda del Rio in Coamo, Puerto Rico was tested for SARS-CoV-2 RNA using the PCR method and the results were negative. A negative test result does not rule out COVID-19 and should not be used as the sole basis for patient management. Due to the high testing volume, results from non-FDA approved collection methods should be interpreted cautiously and extra clinical monitoring may be required. The test was authorized by the FDA under an Emergency Use Authorization.
Original Description:
Original Title
PoZdACqD9eH8rchO1bhhIk4nCbxpat123531 (1)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentLuis Colon-Rivera from Hacienda del Rio in Coamo, Puerto Rico was tested for SARS-CoV-2 RNA using the PCR method and the results were negative. A negative test result does not rule out COVID-19 and should not be used as the sole basis for patient management. Due to the high testing volume, results from non-FDA approved collection methods should be interpreted cautiously and extra clinical monitoring may be required. The test was authorized by the FDA under an Emergency Use Authorization.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
13 views1 pagePo ZD ACq D9 e H8 RCH O1 BHH Ik 4 N Cbxpat 123531
Uploaded by
zhang chaoLuis Colon-Rivera from Hacienda del Rio in Coamo, Puerto Rico was tested for SARS-CoV-2 RNA using the PCR method and the results were negative. A negative test result does not rule out COVID-19 and should not be used as the sole basis for patient management. Due to the high testing volume, results from non-FDA approved collection methods should be interpreted cautiously and extra clinical monitoring may be required. The test was authorized by the FDA under an Emergency Use Authorization.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
ANALYSIS REPORT
Patient Number Birthdate Sex Page
QUEST CAPARRA REFERENCE
Col. Tec. Med. P.R. 9/26/1968 M 1
CAPARRA GALLERY
Pat. No.: 0000197
107 ORTEGON SUITE 103 Paid 5 Cents Physician Name
GUAYNABO, PR 00966-2516 Exp: 2/28/2021
DIRECTOR: MANUEL MARCIAL-SEOANE MD
Num: 001479378 BATISTA-DELFAUS, GUARIONEX
LIC # 060 CLIA # 40D2037283 TEL: (787) 474-2900 FAX: (787) 474-5783 Origin / Special Instructions
016444975 0900-010124-000-0000 010124-DEPT SALUD-FR MUN COAMO
Request 1001479378 Reference Ordered On Supervisor
COLON-RIVERA, LUIS AUG 28,2020 11:54PM MMS
HACIENDA DEL RIO CALLED F 33
COAMO, PR 00769 FIRST RESPONDE Reported On M.T.
TEC LABORATORIO SEP 1,2020 12:21AM AHA
Test Units Results Graphic Normal Range
Molecular Diagnostics
SARS-CoV-2 RNA, PCR......................Negative Negative
****************************************************************************
For Negative Results: The specimen is negative for SARS-CoV-2, the
coronavirus associated with COVID-19. A negative result does not rule out
the possibility of COVID-19 and should not be used as the sole basis for
patient management decisions.
For Positive Results: The specimen is positive for SARS-CoV-2, the
coronavirus associated with COVID-19. Clinical correlation with patient
history and other diagnostics information is necessary to determine patient
infection status.
----------------------------------------------------------------------------
Due to the current public health emergency, Quest Diagnostics is receiving a
high volume of samples from a wide variety of swabs and media for COVID-19
testing. In order to serve patients during this public health crisis,
samples from appropriate clinical sources are being tested. Negative test
results derived from specimens received in non-commercially manufactured
viral collection and transport media, or in media and sample collection kits
not yet authorized by FDA for COVID-19 testing should be cautiously
evaluated and the patient potentially subjected to extra precautions such as
additional clinical monitoring, including collection of an additional
specimen.
This test has been authorized by FDA under an Emergency Use Authorization
(EUA) for use by authorized laboratories.
Please review the “Fact Sheets” for health care providers, and patients and
the FDA authorized labeling available on the Quest website:
www.QuestDiagnostics.com/Covid19
Method: ABBOTT m2000 Real Time SARS-CoV-2 Assay.
ORIGINAL
You might also like
- Covid-19 RT-PCR Laboratory Result Form: City of Dasmariñas Molecular Diagnostic LaboratoryDocument1 pageCovid-19 RT-PCR Laboratory Result Form: City of Dasmariñas Molecular Diagnostic LaboratoryCelineNo ratings yet
- Patient Details Specimen Details Physician DetailsDocument1 pagePatient Details Specimen Details Physician DetailsMax WellsNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- CGH202011008542 Lab-2020-0351447 Laboratory Covid-Pcr-Test PDFDocument2 pagesCGH202011008542 Lab-2020-0351447 Laboratory Covid-Pcr-Test PDFMae SampangNo ratings yet
- BCG#7 COVID 19 BCG Perspectives Version7Document39 pagesBCG#7 COVID 19 BCG Perspectives Version7Isma Madara100% (1)
- DR Vernon Coleman Covid 19 Vaccine - Possible Vaccine Side EffectsDocument2 pagesDR Vernon Coleman Covid 19 Vaccine - Possible Vaccine Side EffectsGeorge KourisNo ratings yet
- Resultadopdf 8Document2 pagesResultadopdf 8algb5105No ratings yet
- Resultadopdf 1 PDFDocument1 pageResultadopdf 1 PDFCarmen CastroNo ratings yet
- Laboratorio Clinico Figueroa: Patient Number Birthdate SexDocument1 pageLaboratorio Clinico Figueroa: Patient Number Birthdate SexCarmen CastroNo ratings yet
- Laboratorio Clinico La 116: Patient Number Birthdate SexDocument1 pageLaboratorio Clinico La 116: Patient Number Birthdate SexVirginia MercadoNo ratings yet
- Resultadopdf 3Document1 pageResultadopdf 3algb5105No ratings yet
- ResultadopdfDocument1 pageResultadopdfCarlos Daniel OrtizNo ratings yet
- Result A DosDocument1 pageResult A DoscdonatovegaNo ratings yet
- Resultadopdf - PHP 2Document2 pagesResultadopdf - PHP 2Leticia Rubio QuilesNo ratings yet
- Resultadopdf 4Document1 pageResultadopdf 4algb5105No ratings yet
- Resultadopdf PHPDocument1 pageResultadopdf PHPnrestofontanezNo ratings yet
- Resultadopdf PHPDocument1 pageResultadopdf PHPMiguel ColonNo ratings yet
- Resultadopdf 2Document1 pageResultadopdf 2algb5105No ratings yet
- Laboratorio Clinico Borinquen: Patient Number Birthdate SexDocument3 pagesLaboratorio Clinico Borinquen: Patient Number Birthdate SexDavid NievesNo ratings yet
- Laboratorio Clinico Parque Escorial: Patient Number Birthdate SexDocument1 pageLaboratorio Clinico Parque Escorial: Patient Number Birthdate SexCarol Melissa AquinoNo ratings yet
- ResultadopdfDocument1 pageResultadopdfOsvaldo TorresNo ratings yet
- Resultadopdf 7Document1 pageResultadopdf 7algb5105No ratings yet
- Laboratorio Clinico Bact. Genesis: Patient Number Birthdate SexDocument1 pageLaboratorio Clinico Bact. Genesis: Patient Number Birthdate SexEVELYN AyalaNo ratings yet
- PML22-003734 - Macatuno, Nicolas Fulgencio - $RT-PCRDocument1 pagePML22-003734 - Macatuno, Nicolas Fulgencio - $RT-PCRDpmmh Lab DeptNo ratings yet
- T2100001419 ML2100001252 115620 6691600 19370729 $mole-DefauDocument2 pagesT2100001419 ML2100001252 115620 6691600 19370729 $mole-DefauPeony03No ratings yet
- T2100001419 ML2100001252 115620 6691600 19370729 $mole-DefauDocument2 pagesT2100001419 ML2100001252 115620 6691600 19370729 $mole-DefauPeony03No ratings yet
- ResultadopdfDocument2 pagesResultadopdfalgb5105No ratings yet
- CGH202012022479 Lab-2020-0411918 Laboratory Covid-Pcr-TestDocument2 pagesCGH202012022479 Lab-2020-0411918 Laboratory Covid-Pcr-TestJosa Camille BungayNo ratings yet
- Vit - B-12 2Document1 pageVit - B-12 2ralaro177No ratings yet
- Laboratory Result Form: Pontilar, Gretchel CondinoDocument1 pageLaboratory Result Form: Pontilar, Gretchel CondinoGretchel PontilarNo ratings yet
- ResultadopdfDocument1 pageResultadopdfalfredo velezNo ratings yet
- Kapuno, Natalie EveDocument1 pageKapuno, Natalie EveVee KeeNo ratings yet
- Pathology & Clinical Laboratory (M) SDN - BHD: Scan QR For VerificationDocument2 pagesPathology & Clinical Laboratory (M) SDN - BHD: Scan QR For VerificationAdnan Md SaatNo ratings yet
- Manoriã - A, ElmaDocument1 pageManoriã - A, ElmaElmaNo ratings yet
- CGH202011011832 Lab-2020-0356025 Laboratory Covid-Pcr-TestDocument2 pagesCGH202011011832 Lab-2020-0356025 Laboratory Covid-Pcr-TestJhon Rosete ParicoNo ratings yet
- CGH202008000915 - Lab A2 2020 2231 - Laboratory - Covid PCR Test PDFDocument2 pagesCGH202008000915 - Lab A2 2020 2231 - Laboratory - Covid PCR Test PDFMichael JonasanNo ratings yet
- Apatan, John Carlo SenaderoDocument1 pageApatan, John Carlo SenaderoJOHN CARLO APATANNo ratings yet
- Corpuz Betty AlcantaraDocument1 pageCorpuz Betty AlcantaraAbn Pop UpNo ratings yet
- 22-007272 - T2200007270 - 2022-10598 - Taca, Lalaine C. - 04262022120622-10598 - 6 - 0 - 19910327 - RT-PCRDocument2 pages22-007272 - T2200007270 - 2022-10598 - Taca, Lalaine C. - 04262022120622-10598 - 6 - 0 - 19910327 - RT-PCRAmro ShalabiNo ratings yet
- ResultadopdfDocument1 pageResultadopdfEvelyn ValentinNo ratings yet
- Client Information: Quest Caparra ReferenceDocument1 pageClient Information: Quest Caparra ReferenceelvisNo ratings yet
- Laboratorio Clinico Michelsan: Patient Number Birthdate SexDocument1 pageLaboratorio Clinico Michelsan: Patient Number Birthdate SexDaniel CadizNo ratings yet
- LabResultTempPDF CJ0304865Document2 pagesLabResultTempPDF CJ0304865Jahred EstebanNo ratings yet
- MUST To KNOW in Clinical ChemistryDocument1 pageMUST To KNOW in Clinical ChemistryEdel BinasoyNo ratings yet
- Coreplus Serv. Clínicos Y Patológicos: Patient Number Birthdate SexDocument1 pageCoreplus Serv. Clínicos Y Patológicos: Patient Number Birthdate Sexjmcc1983No ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedDocument1 pageSars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedKaoruTecsonNo ratings yet
- PML23 000845 - Soriano Maria Corazon Muñoz - RT PCR 1Document1 pagePML23 000845 - Soriano Maria Corazon Muñoz - RT PCR 1Dpmmh Lab DeptNo ratings yet
- Captura de Pantalla 2022-05-26 A La(s) 9.09.50 A. M.Document1 pageCaptura de Pantalla 2022-05-26 A La(s) 9.09.50 A. M.Madelyn Diaz RamosNo ratings yet
- PML22-001509 - Bernabe, Aurora Ocasla - $RT-PCRDocument1 pagePML22-001509 - Bernabe, Aurora Ocasla - $RT-PCRDpmmh Lab DeptNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- ResultadopdfDocument1 pageResultadopdfArielNo ratings yet
- RTPCR ReportDocument1 pageRTPCR ReportDhruvin KapadiaNo ratings yet
- Resultadopdf 6Document2 pagesResultadopdf 6algb5105No ratings yet
- Department of Molecular Biology: Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology: Test Name Result Unit Bio. Ref. Range MethodKaran GuptaNo ratings yet
- Philippine Red Cross Molecular Laboratory Result Form: Date: NameDocument1 pagePhilippine Red Cross Molecular Laboratory Result Form: Date: NameJohn De VillaNo ratings yet
- Molecular Biological Test: Uriarte Rojo, DavidDocument1 pageMolecular Biological Test: Uriarte Rojo, DavidDavid Uriarte RojoNo ratings yet
- Acelar Robert Gueatelara 7Document2 pagesAcelar Robert Gueatelara 7Robert AcelarNo ratings yet
- Resultadopdf2 PHPDocument1 pageResultadopdf2 PHPMelvin RuizNo ratings yet
- Macario, Angeline PedoyDocument1 pageMacario, Angeline PedoyAngeline MacarioNo ratings yet
- Ms MANNATDocument1 pageMs MANNATMannat KaundalNo ratings yet
- LG23 566175Document1 pageLG23 566175Airo Nikko SolpicoNo ratings yet
- 42.parth Sanjay SalunkeDocument10 pages42.parth Sanjay SalunkeJunaidNo ratings yet
- Activity#3: Answer The Following QuestionDocument2 pagesActivity#3: Answer The Following QuestionMark Roland Caceres100% (1)
- CIR NO 035 - Grade 6 Reopening CircularDocument6 pagesCIR NO 035 - Grade 6 Reopening CircularSaroja RajuNo ratings yet
- Face Masks: Benefits and Risks During The COVID-19 Crisis: European Journal of Medical ResearchDocument8 pagesFace Masks: Benefits and Risks During The COVID-19 Crisis: European Journal of Medical ResearchgrowlingtoyouNo ratings yet
- Mary Oliver - US History Final ProjectDocument6 pagesMary Oliver - US History Final ProjectMary OliverNo ratings yet
- Moderna CEO Stephane Bancel Says Coronavirus Vaccine Price Will Be LowDocument3 pagesModerna CEO Stephane Bancel Says Coronavirus Vaccine Price Will Be Lowtp4oyk fdtaz4100% (1)
- Iga - PmppaDocument38 pagesIga - PmppaRapplerNo ratings yet
- Repponse To Motion For Release 37Document14 pagesRepponse To Motion For Release 37the kingfishNo ratings yet
- Dr. Dian Nurul Al Amini, SP - THTDocument6 pagesDr. Dian Nurul Al Amini, SP - THTRifaniNugrohoNo ratings yet
- Orca - Share - Media1623712617928 - 6810344328229419580 (Repaired)Document21 pagesOrca - Share - Media1623712617928 - 6810344328229419580 (Repaired)lorejane aseoNo ratings yet
- CDC Interim Reopening GuidanceDocument62 pagesCDC Interim Reopening GuidanceAlex GeliNo ratings yet
- Coronavirus Will See Its End Soon, Say Renowned Astrologers - The WeekDocument4 pagesCoronavirus Will See Its End Soon, Say Renowned Astrologers - The WeekManuNo ratings yet
- Covid EssayDocument2 pagesCovid EssayThant Htet SintNo ratings yet
- PSMID COVID TX Guidelines V.3.31.20a PDFDocument62 pagesPSMID COVID TX Guidelines V.3.31.20a PDFRenz Marion AlemaniaNo ratings yet
- Arizona Ruling On Lawsuit Against Gym ClosuresDocument24 pagesArizona Ruling On Lawsuit Against Gym ClosuresKTARNo ratings yet
- A. Muh. Alif Rumansyah - 185020307111031 - THE IMPACT OF CORONA VIRUS IN INDIADocument10 pagesA. Muh. Alif Rumansyah - 185020307111031 - THE IMPACT OF CORONA VIRUS IN INDIAalif rumansyahNo ratings yet
- University Students Online Learning System During Covid-19 Pandemic: Advantages, Constraints and SolutionsDocument7 pagesUniversity Students Online Learning System During Covid-19 Pandemic: Advantages, Constraints and SolutionsIssam BoujnaneNo ratings yet
- Test Report: MR - Raghavan Venkatraman (39/M)Document2 pagesTest Report: MR - Raghavan Venkatraman (39/M)Raghavan VenkatramanNo ratings yet
- M1.1. Korea Experience - Flattening The Curve On COVID-19 PDFDocument90 pagesM1.1. Korea Experience - Flattening The Curve On COVID-19 PDFDax Kin Dy VintolaNo ratings yet
- Toyota Alabang, Incorporated Covid-19 Manual For Continuous Work ScenarioDocument8 pagesToyota Alabang, Incorporated Covid-19 Manual For Continuous Work ScenarioRon PascualNo ratings yet
- 5 Awesome Pinoy Scientists: Angel C. AlcalaDocument6 pages5 Awesome Pinoy Scientists: Angel C. AlcalaZumainah Bato AliNo ratings yet
- Claudious Gwaze AssignmentDocument14 pagesClaudious Gwaze AssignmentStandrick MuchiniNo ratings yet
- COVID-19 Preventive & Mitigation Action PlanDocument14 pagesCOVID-19 Preventive & Mitigation Action PlanSaikat DawanNo ratings yet
- Kohler-Wright2020 Article TheUrgentNeedForTransparentAndDocument4 pagesKohler-Wright2020 Article TheUrgentNeedForTransparentAndRoshanNo ratings yet
- Health Surveilance For VibrationDocument9 pagesHealth Surveilance For VibrationNishadh NishNo ratings yet
- Et Al.,: Defendants' Memorandum in Opposition To Plaintiff'S Motion For A Preliminary InjunctionDocument46 pagesEt Al.,: Defendants' Memorandum in Opposition To Plaintiff'S Motion For A Preliminary Injunctionnate_raymondNo ratings yet
- Jon Rappoport's Blog: Corona: Creating The Illusion of A Pandemic Through Diagnostic TestsDocument24 pagesJon Rappoport's Blog: Corona: Creating The Illusion of A Pandemic Through Diagnostic TestsDonnaveo ShermanNo ratings yet
- The Origin of SARS-CoV-2Document10 pagesThe Origin of SARS-CoV-2habomuninNo ratings yet