Professional Documents
Culture Documents
Paper-4: HG SL HSG ZN 2Hcl ZNCL H 2naoh H So Na So 2ho
Paper-4: HG SL HSG ZN 2Hcl ZNCL H 2naoh H So Na So 2ho
Uploaded by
subhaseduOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Paper-4: HG SL HSG ZN 2Hcl ZNCL H 2naoh H So Na So 2ho
Paper-4: HG SL HSG ZN 2Hcl ZNCL H 2naoh H So Na So 2ho
Uploaded by
subhaseduCopyright:
Available Formats
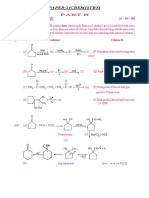
PAPER-4
1. Match the Column I and Column II
(A) H3PO2 (p) Dibasic
(B) H3PO 4 (q) Monobasic
(C) H3PO3 (r) Tribasic
(D) H3BO3 (s) Aprotic
2. Match the Column I and Column II
(A) Washing soda (p) Hasenclever plant
(B) Bleaching powder (q) Chlor-alkali process
(C) Caustic soda (r) Solvay process
(D) Baking soda (s) Castner and kellner cell
3. Match the following
Column – I Column – II
(A) NaHCO3 (P) Baking soda
(B) NaOH (Q) Alkaline
(C) KHSO4 (R) Acidic salt
(D) Ca(OH)2 (S) Bitter taste
4. Match the following
Column – I Column – II
(A)
KClO3 (P) O2
(B)
ZnCO3 (Q) H2O
(C)
H 2 CO3 (R) CO2
(D)
C2 H 6 (S) ZnO
5. Match the following
Column – I Column – II
(A) H2 g S l
H2 S g (P) Displacement reaction
,boiling
(B) Zn 2HCl ZnCl2 H2 (Q) Neutralization reaction
(C) 2NaOH H2 SO 4 Na 2 SO 4 2H2 O (R) Decomposition reaction
(D) 2Cu NO3 2
2CuO 4NO2 O2 (S) Combination reaction
PAPER-4
6. Match the Column – I with Column – II
Column – I Column – II
(A) HCl (P) Strong acid
(B) HCN (Q) Weak acid
(C) NaOH (R) Weak base
(D) NH4OH (S) Strong base
(E) Distilled water (T) Neutral
7. Match the following
Column – I Column – II
A) Plaster of paris p) CaSO4.2H2O
B) Baking soda q) Na2CO3.10H2O
C) Washing Soda r) CaSO4. ½ H2O
D) Gypsum s) NaHCO3
8. Match the following
Column – II (Number of
Column – I (Redox Change)
electrons involved in change)
A) MnO2 Mn2O3 p) 4
B) MnO2 MnSO4 q) 2
C) MnO2 Mn r) 3
D) KMnO4 MnO2 s) 1
9. Match the acid/base in Column –I with their nature in Column -II
Column-I Column-II
(A) HSO 4 (p) Lewis acid
(B) BF3 (q) Lewis base
(C) NH3 (r) Bronsted acid
(D) OH (s) Bronsted base
PAPER-4
10. Match the compound in Column –I with their oxidation state in Column -II
Column-I Column-II
(A) N 2 O5 (p) N=2
(B) NH3 (q) O=1
(C) H 2O 2 (r) H=+1
(D) N2H4 (s) N=5
(t) N=3
11. Match the following
Column – I Column – II
A) HSO4 p) Lewis acid
B) BF3 q) Lewis base
C) NH3 r) Bronsted acid
D) OH s) Bronsted base
12. Match the following
Column – II (Chemical
Column – I (Redox Change)
equations)
A) Combination reaction p) CaCO3 CaO + CO2
B) Decomposition reaction q) 2H2O 2H2+ O2
C) Displacement reaction r) CaO + CO2 CaCO3
D)
Electrolysis reaction
s) Fe+CuSO4 (aq.)
FeSO4(aq)+Cu
You might also like
- Chemistry 11th Edition Chang Test BankDocument20 pagesChemistry 11th Edition Chang Test BankRobertSmithfpdzw100% (17)
- ICSE History and CivicsDocument6 pagesICSE History and CivicssubhaseduNo ratings yet
- Little Match GirlDocument8 pagesLittle Match GirlsubhaseduNo ratings yet
- Biomolecules, Polymers, Poc: Matching Answer Type QuestionsDocument12 pagesBiomolecules, Polymers, Poc: Matching Answer Type Questionssree anugraphicsNo ratings yet
- Class 12 Chemistry Ch-8.Aldehydes, Ketones and Carboxylic AcidsDocument53 pagesClass 12 Chemistry Ch-8.Aldehydes, Ketones and Carboxylic Acidskingoo0f1No ratings yet
- Fiitjee Class X Practice Worksheet Organic Chemistry-2Document2 pagesFiitjee Class X Practice Worksheet Organic Chemistry-2T3X1CNo ratings yet
- Aldehyde and KetonesDocument5 pagesAldehyde and KetonesEswara ReddyNo ratings yet
- GR 1Document6 pagesGR 1Sipra PaulNo ratings yet
- Chemistry Kcet 2023Document9 pagesChemistry Kcet 2023Poorni RenuNo ratings yet
- More Than One Option Correct 1Document4 pagesMore Than One Option Correct 1AryanNo ratings yet
- CH - 2 Worksheet 2Document6 pagesCH - 2 Worksheet 2HarshNo ratings yet
- Class Test-8 Biomolecules JEE Adv CC AnsDocument4 pagesClass Test-8 Biomolecules JEE Adv CC Ansbruh pogNo ratings yet
- HydrocarbonDocument7 pagesHydrocarbonEswara ReddyNo ratings yet
- Exercise - V (Matrix) : O OH H CH OhDocument2 pagesExercise - V (Matrix) : O OH H CH OhAnant Preet SinghNo ratings yet
- Selina Concise Chemistry Class 9 ICSE Solutions For Chapter 1 - Language of ChemistryDocument24 pagesSelina Concise Chemistry Class 9 ICSE Solutions For Chapter 1 - Language of ChemistryfelixNo ratings yet
- Goc-Sheet-4-Acidity & BasicityDocument7 pagesGoc-Sheet-4-Acidity & BasicityAaRaV KuShWaHaNo ratings yet
- CHEMISTRY - (12th & 13th) (POI) Paper 2Document7 pagesCHEMISTRY - (12th & 13th) (POI) Paper 2Raju SinghNo ratings yet
- Chemistry 5Document5 pagesChemistry 5Subhra BiswasNo ratings yet
- ACA-3B Full Inorganic Chemistry Class (11+12) (152 Questions+Answers)Document16 pagesACA-3B Full Inorganic Chemistry Class (11+12) (152 Questions+Answers)Biswajit GhoshNo ratings yet
- UntitledDocument10 pagesUntitledAnant M NNo ratings yet
- Redox ReactionsDocument10 pagesRedox ReactionsthilaivananNo ratings yet
- Carbonyl Compound CPP-1Document12 pagesCarbonyl Compound CPP-1UtsavNo ratings yet
- 56 Paper: Iit-Jam 2012Document7 pages56 Paper: Iit-Jam 2012prakhar vishwakarmaNo ratings yet
- GRP 15 To 18 QuestionDocument17 pagesGRP 15 To 18 QuestionKartik YadavNo ratings yet
- P Block 1Document19 pagesP Block 1Sambhav Singhal100% (1)
- P Block Live Class-3 Teacher Notes - RemovedDocument4 pagesP Block Live Class-3 Teacher Notes - RemovedJee AspirantNo ratings yet
- Chemical Bonding 3Document3 pagesChemical Bonding 3Anonymous vRpzQ2BLNo ratings yet
- 17 - D-F BlockDocument4 pages17 - D-F BlockTejas DalwadiNo ratings yet
- Single Answer Type Questions:: (D) Absorption of Light by The Solvated ElectronsDocument4 pagesSingle Answer Type Questions:: (D) Absorption of Light by The Solvated Electronssree anugraphicsNo ratings yet
- Acid & Amine-QuestionDocument6 pagesAcid & Amine-QuestionAnurag RamachandranNo ratings yet
- Heating: So H - Conc) U (. Compd Agno Naoh - LN So NH HCL - Dil NaohDocument4 pagesHeating: So H - Conc) U (. Compd Agno Naoh - LN So NH HCL - Dil NaohVanshaj GuptaNo ratings yet
- Qttsop: 1Os12Thzsoytlhdoztbasoy - Yphyyyyip.ODocument10 pagesQttsop: 1Os12Thzsoytlhdoztbasoy - Yphyyyyip.O33-Siddharth NairNo ratings yet
- KCET ChemDocument10 pagesKCET ChemHarshNo ratings yet
- Road Map (3) Problem: Aq. KOHDocument1 pageRoad Map (3) Problem: Aq. KOHShubham RajNo ratings yet
- 03 Roadmap Oc Eng Student Copy 1595834497 PDFDocument1 page03 Roadmap Oc Eng Student Copy 1595834497 PDFsantosh tripathyNo ratings yet
- 03 Roadmap Oc Eng Student Copy 1595834497 PDFDocument1 page03 Roadmap Oc Eng Student Copy 1595834497 PDFsantosh tripathyNo ratings yet
- Redox Reactions-T-5Document2 pagesRedox Reactions-T-5Soham SagaonkarNo ratings yet
- P Block2Document25 pagesP Block2Vanshika MittalNo ratings yet
- DPP1 SBlock Advan6264893396548698825Document4 pagesDPP1 SBlock Advan6264893396548698825Drushya SalunkeNo ratings yet
- P Block ElementsDocument10 pagesP Block ElementsEzhil MukilNo ratings yet
- Aldehydes and Ketones (Questions)Document27 pagesAldehydes and Ketones (Questions)Dhruv KuchhalNo ratings yet
- Time: 1 Hrs Max. Marks: 98 Single Correct: 3 2 4 HG ZNDocument6 pagesTime: 1 Hrs Max. Marks: 98 Single Correct: 3 2 4 HG ZNlakshmi.vedanarayanan7785No ratings yet
- Balancing of Redox Reactions - 1Document2 pagesBalancing of Redox Reactions - 1Anonymous vRpzQ2BL0% (1)
- Chemistry: ONLY ONE Is CorrectDocument6 pagesChemistry: ONLY ONE Is CorrectSonalNo ratings yet
- 30 Daily Tutorial SheetDocument8 pages30 Daily Tutorial SheetMeera SarangapaniNo ratings yet
- Lesson 1 ChemistryDocument41 pagesLesson 1 Chemistry359 Srinivasa RamanNo ratings yet
- 10 # AllotropesDocument4 pages10 # Allotropesbbooga603No ratings yet
- Dhruv Tuition Classes Class-X Science Term-1 Sample Paper 2Document13 pagesDhruv Tuition Classes Class-X Science Term-1 Sample Paper 2Nisha SinghNo ratings yet
- Chemical Reactions Practice Test 75/75Document4 pagesChemical Reactions Practice Test 75/75Irina StefaniaNo ratings yet
- 50 Expected QuestionsDocument6 pages50 Expected QuestionsShadhasanNo ratings yet
- Aromatic Compounds 13thDocument15 pagesAromatic Compounds 13thRaju SinghNo ratings yet
- The Tine Giren at The Head of This Paper Is The Time Allotted For Writing The AnswersDocument8 pagesThe Tine Giren at The Head of This Paper Is The Time Allotted For Writing The Answersannettedenny4No ratings yet
- Salt AnalysisDocument17 pagesSalt Analysisvijaylakshmi0727No ratings yet
- Prelims 1 ICSE Dec 2023Document6 pagesPrelims 1 ICSE Dec 2023kuldeep9034.patelNo ratings yet
- 2023 ChemistryDocument6 pages2023 Chemistrysumitali7638No ratings yet
- Time: 1 Hrs Max. Marks: 75 Single Correct: - O OH CH CH NH C Me - ODocument5 pagesTime: 1 Hrs Max. Marks: 75 Single Correct: - O OH CH CH NH C Me - Olakshmi.vedanarayanan7785No ratings yet
- P Block Elements (Q.B) 13thDocument6 pagesP Block Elements (Q.B) 13thRaju SinghNo ratings yet
- Dec - 2012Document20 pagesDec - 2012Sumanta- 14No ratings yet
- Acidic Basic StrengthDocument12 pagesAcidic Basic StrengthDEV SHARMANo ratings yet
- Redox Reactionstest PDFDocument1 pageRedox Reactionstest PDFaleena'No ratings yet
- Reaction Mechnism-1Document6 pagesReaction Mechnism-1Harshil rawalNo ratings yet
- IJRHS 2016 Vol04 Issue 04 10Document8 pagesIJRHS 2016 Vol04 Issue 04 10subhaseduNo ratings yet
- Neutral Control and CoordinationDocument114 pagesNeutral Control and CoordinationsubhaseduNo ratings yet
- Maths Term 1 - QPs 1,2,3Document12 pagesMaths Term 1 - QPs 1,2,3subhaseduNo ratings yet
- Project CoverDocument5 pagesProject CoversubhaseduNo ratings yet
- Cover Page 2 Cbse 2024Document1 pageCover Page 2 Cbse 2024subhaseduNo ratings yet
- Cover Page-1 Cbse 24Document1 pageCover Page-1 Cbse 24subhaseduNo ratings yet
- Test 3Document42 pagesTest 3subhaseduNo ratings yet
- IBEF # Oil-and-Gas-January-2021Document44 pagesIBEF # Oil-and-Gas-January-2021subhaseduNo ratings yet
- Formatted Rules For Prepositions 19th MayDocument2 pagesFormatted Rules For Prepositions 19th MaysubhaseduNo ratings yet
- CISCE - ICSE - 2018-2019 - March - English 2 (Literature in English)Document14 pagesCISCE - ICSE - 2018-2019 - March - English 2 (Literature in English)subhaseduNo ratings yet
- Materials of The Earth's Crust - Rocks Note 5Document3 pagesMaterials of The Earth's Crust - Rocks Note 5subhaseduNo ratings yet
- CISCE - ICSE (Specimen) - 2018-2019 - March - English 1 (English Language)Document7 pagesCISCE - ICSE (Specimen) - 2018-2019 - March - English 1 (English Language)subhaseduNo ratings yet
- Preposition Exercise q1 AnswerDocument1 pagePreposition Exercise q1 Answersubhasedu100% (1)
- Test 4Document43 pagesTest 4subhaseduNo ratings yet
- Class9A, B, C:Physi CS:NOTE6:: Der I Vat I Onoft Heequat I Onsofmot I OnDocument3 pagesClass9A, B, C:Physi CS:NOTE6:: Der I Vat I Onoft Heequat I Onsofmot I OnsubhaseduNo ratings yet
- All Summer in A DayDocument8 pagesAll Summer in A Daysubhasedu100% (1)
- NEET Books For PhysicsDocument1 pageNEET Books For PhysicssubhaseduNo ratings yet
- Biology Chapter 4: The Flower Lesson-1 Class-9A: Stalk (Pedicel) Without A Stalk (Sessile) Receptacle Thalamus TDocument6 pagesBiology Chapter 4: The Flower Lesson-1 Class-9A: Stalk (Pedicel) Without A Stalk (Sessile) Receptacle Thalamus TsubhaseduNo ratings yet
- Class9A, B, C:Physi CS:NOTE5:: Aver AgespeedDocument4 pagesClass9A, B, C:Physi CS:NOTE5:: Aver AgespeedsubhaseduNo ratings yet
- KVPY SyllabusDocument4 pagesKVPY SyllabussubhaseduNo ratings yet
- Math Exam 18 Icse 10Document158 pagesMath Exam 18 Icse 10subhasedu100% (1)
- L-2:Structure of Chromosomes, Cell Cycle and Cell Division. L-3:Genetics - Some Basic Fundamentals L-4: Absorption by Roots, L - 5: TranspirationDocument7 pagesL-2:Structure of Chromosomes, Cell Cycle and Cell Division. L-3:Genetics - Some Basic Fundamentals L-4: Absorption by Roots, L - 5: TranspirationsubhaseduNo ratings yet
- How Far Is The River Page 9Document100 pagesHow Far Is The River Page 9subhaseduNo ratings yet
- Comparative Analysisof Rateof InterestDocument13 pagesComparative Analysisof Rateof InterestsubhaseduNo ratings yet
- ICSE BiologyDocument10 pagesICSE BiologysubhaseduNo ratings yet
- ICSE GeographyDocument7 pagesICSE Geographysubhasedu0% (1)
- Cls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Document34 pagesCls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Jotiraj Parihar50% (2)
- CombinepdfDocument14 pagesCombinepdfBee Jay JayNo ratings yet
- O Chem 221Document1 pageO Chem 221jennawallaceNo ratings yet
- Lecture 7 Polyprotics Acids, Titration Curves, BuffersDocument63 pagesLecture 7 Polyprotics Acids, Titration Curves, BuffersYahmeela SernaNo ratings yet
- FatsDocument2 pagesFatsblueberry eyesNo ratings yet
- Formal Report-Proteins and Amino AcidsDocument10 pagesFormal Report-Proteins and Amino AcidsQuenieMarielIlar100% (1)
- A Textbook of Organic Chemistry 1923Document935 pagesA Textbook of Organic Chemistry 1923Basharat RasheedNo ratings yet
- MCAT Organic Summary SheetDocument6 pagesMCAT Organic Summary SheetSpencer Thomas100% (2)
- Experiment 2: Complexometric Titration 1.0 ObjectivesDocument4 pagesExperiment 2: Complexometric Titration 1.0 ObjectivesSangetha ChelladoraiNo ratings yet
- NTA ABHYAS I P-Block Elements I VERMA SIRDocument5 pagesNTA ABHYAS I P-Block Elements I VERMA SIRarslaan8799No ratings yet
- Worksheet 2.2 Ions and Naming Compounds: Given Ion SymbolDocument2 pagesWorksheet 2.2 Ions and Naming Compounds: Given Ion SymbolZach CariñoNo ratings yet
- Chemistry Deleted and Added Portion For JEE Main 2024Document2 pagesChemistry Deleted and Added Portion For JEE Main 2024cᴘcтԍᴀмιɴԍ YTNo ratings yet
- Chem 224-Spring-2017-Schedule PDFDocument8 pagesChem 224-Spring-2017-Schedule PDFRichard OletskyNo ratings yet
- HEDP - Qualitative Complexing AgentsDocument6 pagesHEDP - Qualitative Complexing AgentsMohammad MariasaNo ratings yet
- How To Identify Geometrical Isomers by S.K.sinha See Chemistry Animations atDocument4 pagesHow To Identify Geometrical Isomers by S.K.sinha See Chemistry Animations atmyiitchemistry100% (4)
- Organohalides: Based On Mcmurry'S, 7 EditionDocument32 pagesOrganohalides: Based On Mcmurry'S, 7 EditionmaherNo ratings yet
- (SSC) Consumer Chemistry9 Q1 M6 W6Document24 pages(SSC) Consumer Chemistry9 Q1 M6 W6.No ratings yet
- Jyothish Kumar Took Sulphur Powder On A Spatula and Heated It. HeDocument9 pagesJyothish Kumar Took Sulphur Powder On A Spatula and Heated It. HeSharan SiuuNo ratings yet
- CST 01 QPDocument18 pagesCST 01 QPmidhatsyedpukhtaNo ratings yet
- CHM 211 AssignmentDocument2 pagesCHM 211 AssignmentJusila GNo ratings yet
- Organic Synthesis TEMPLATEDocument30 pagesOrganic Synthesis TEMPLATEKyle HallNo ratings yet
- Functional Group TestsDocument1 pageFunctional Group Testsnalla suhasNo ratings yet
- IUPAC Name 18th Sep 15Document8 pagesIUPAC Name 18th Sep 15samarthNo ratings yet
- Chemistry 074478 File1of12Document106 pagesChemistry 074478 File1of12Matthew CullNo ratings yet
- BOOKLET - Chemsheets A2 009 (Acids - Bases)Document21 pagesBOOKLET - Chemsheets A2 009 (Acids - Bases)sophieNo ratings yet
- Bcids, Bases and Salts: To Purchase Hard Book of From Amazon Click HereDocument41 pagesBcids, Bases and Salts: To Purchase Hard Book of From Amazon Click HereSarvesh Kumar100% (1)
- Intermolecular Forces: Non-Polar Covalent Bond (Formed by Equal Sharing of Electrons) and (3) Metallic Bond IsDocument7 pagesIntermolecular Forces: Non-Polar Covalent Bond (Formed by Equal Sharing of Electrons) and (3) Metallic Bond IsSharon May JavierNo ratings yet
- Experiment 9 Formal ReportDocument5 pagesExperiment 9 Formal ReportTrishaNo ratings yet
- Stoichiometry 2 QPDocument10 pagesStoichiometry 2 QPYee MeiNo ratings yet