Professional Documents
Culture Documents
Organic Chemistry 2: (I) RMGBR (Ii) H /H O
Uploaded by
arielOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organic Chemistry 2: (I) RMGBR (Ii) H /H O
Uploaded by
arielCopyright:
Available Formats
Organic Chemistry 2
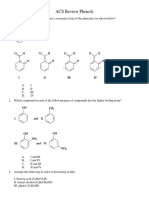
1. Which of the following compounds would NOT be 10. What is the position of the substituent after the
considered aromatic? nitration of benzoic acid?
a. Para position
b. Either ortho or para position
c. Meta position
d. Ortho position
a. c.
11. A primary alcohol under the reagent sodium dichromate
under acidic condition and water produces which of
the following?
a. An aldehyde c. A carboxylic acid
b. d. b. A ketone d. A quinone
2. Which among the choices is NOT aromatic? 12. What is the reactant needed to produce 3-methyl-2-
pentene when reacted with hot sulfuric acid?
H H a. 2-pentyne c. 3-methyl-2-pentanone
b. 3-methyl-2-pentanol d. 3-methylpentanal
For numbers 13-15, consider the following:
a. c.
H a. 2-pentanol c. 3-methyl-3-pentanol
b. 2-methyl-1-pentanol d. None of the above
13. Which alcohol would react quickly with Lucas reagent
(equimolar amounts of HCl and ZnCl2)?
14. Which alcohol would be oxidized to an aldehyde upon
b. d. reacting with a weak oxidizing agent?
15. Which alcohol would be oxidized to a ketone upon
3. Which of the following is/are characteristics of an reaction with Jones reagent?
aromatic compound?
I. It is cyclic and planar. 16. What is the IUPAC name of the product from the
II. It is completely conjugated. reaction given?
III. It is composed of even-numbered carbon atoms.
HBr
a. I only c. I and III ???
0°C
b. I and II d. I, II and III
a. 3-methyl-2-hexene c. 3-methyl-3-hexene
4. Which of the following substituents is NOT an ortho- b. 3-bromo-3-methylhexane d. 3-methylhexane
para director in an EAS reaction?
a. -Cl c. -OH 17. Which R group would complete the Grignard reaction
b. -NO2 d. -CH3 given?
5. Which of the following describes the effect of a
nitro group (-NO2) as a substituent in EAS? (i) RMgBr
a. Weakly activating, meta directing
b. Strongly deactivating, meta directing (ii) H+/H2O
c. Strongly activating, ortho-para directing
d. Weakly deactivating, ortho-para directing a. Sec-butyl c. propyl
b. Butyl d. isopropyl
6. What is the major organic product from the following
reaction? 18. What is the product when 2-propanol reacts with
sodium dichromate in acidic condition?
a. Propanone c. Propanoic acid
HNO3 b. Propanal d. Ethanoic acid
???
H2SO4
19. Which of the following will be useful to determine
a. m-nitrotoluene only c. o-nitrotoluene only the aromaticity of a compound?
b. p-nitrotoluene only d. o-nitrotoluene and a. 4n + 2 c. 4n + 2π
p-nitrotoluene b. n = 2π/4n d. n = 4n + 2
7. What reacts with benzene to produce a benzoic acid? 20. How many π electrons are present in the given
a. CH3Cl with AlCl3 and hot KMnO4 with water under compound?
acidic condition
b. CH3Cl with AlCl3 and excess NBS
c. CH3Cl with AlCl3 and hot Zn(Hg) in acidic
condition
d. Ethanoic acid with AlCl3
a. 4 c. 8
8. What is the first step in producing 4- b. 10 d. 12
bromobenzonitrile from benzene?
a. Nitration the reduction using Fe or Zn 21. The reaction given proceeds to form which of the
b. Nitration following?
c. Acylation then bromination O O
d. Bromination
OH 1) LAH, ether
9. What is the last step in producing 4-bromo-2- ???
nitrotoluene from benzene? 2) H2O
a. Bromination
b. Nitration a. Two carboxylic acid group
c. Friedel-Crafts alkylation b. An aldehyde and a ketone
d. Friedel-Crafts acylation c. A diol
d. An aldehyde to the carboxylic acid substituent
Prepared by: Engr. Quennie Marie L. Bañares
Organic Chemistry 2
22. The reaction given proceeds to form which of the 29. Which of the following is the product from the
following? reaction given?
OH PCC (1) EtMgBr
??? ???
CH2Cl2 (2) H2O
HO a. 2-methylpentanol c. 1-methylpentanol
b. 2-methyl-2- pentanol d. isopropyl-2-pentanol
30. Which of the following is used to prepare a sulfide
a. c.
from an alkyl halide compound?
a. An alkyl halide in basic condition
b. Sodium hydrosulfide
c. An alkyl halide
d. NaIO4
b. d.
31. What is the description of the compound given?
23. What is the common name of the ether given below?
R R’
a. A Sulfone c. A Sulfide
a. 2-ethoxypropane
b. A Sulfoxide d. A Sulfonoxide
b. 2-propoxy ethane
c. Ethyl isopropyl ether
32. Among the choices given, which would break a
d. Isopropyl ethyl ether
disulfide bond?
a. A strong base with Zn metal catalyst
24. Which of the following is TRUE about ethers?
b. Any strong base
a. Ethers are completely soluble in water.
c. Any strong acid
b. Ethers are reactive towards strong acids.
d. A strong acid with Zn metal catalyst
c. Ethers are not soluble in organic solvents.
d. Ethers form colored complexes with Fe3+
33. Which among the choices is true with the reaction
given?
25. Which of the following compound represents 1,2-
epoxycyclohexane? (1) Hg(OAc)2
(2) NaBH4
a. It undergoes an oxymercuration-demercuration
reaction.
b. It lacks 2-propanol to complete the reaction.
a. c. c. The reagent used to complete the reaction is
H3C wrong. It should undergo hydroboration-oxidation.
d. The reaction cannot happen without a strong acid.
34. Which among the choices is correct?
a. An alkoxymercuration-demercuration undergoes
b. d. substitution reaction.
b. An alkoxymercuration-demercuration undergoes
26. The reaction for the epoxide given started with an elimination reaction.
alkyne forming an alkene. Which among the choices is c. An alkoxymercuration-demercuration undergoes a
the reaction of alkene that would result to the given Markovnikov addition.
epoxide? d. An alkoxymercuration-demercuration undergoes an
anti-Markovnikov addition.
35. Which among the choices is the IUPAC name for the
compound given?
a. Sodium under ammonia solution CH3
b. Hydrogen with a poisonous catalyst
c. Either A or B would result to the same epoxide
CH3CHCH2CH2SH
d. NaNH2 with an alkyl halide
a. 3-methyl-1-butanethiol
27. Which of the following represents the systematic name b. 3-methyl-1-butanesulfide
for the given compound? c. 1-thiol-1,1-dimethylpropane
d. 1-sulfide-1,1-dimethylpropane
36. What is needed to produce sulfone from sulfoxide?
a. NaOH c. NaIO4
a. Butoxypropane c. 1-methylpropylpropane b. HCl d. Hydrogen peroxide
b. 1-methylethoxybutane d. 2-ethoxybutane
37. What is needed to produce sulfoxide from sulfides?
28. Which of the following is the structure for ethyl c. NaOH c. NaIO4
propyl sulfide? d. HCl d. Hydrogen peroxide
SH
38. An alkyne could be reacted to form a ketone using
which of the following reagents?
a. c. a. PCC/CH2Cl2 c. Na2Cr2O7/H2SO4, H2O
b. H2SO4, H2O/HgSO4 d. Wittig reaction
39. Aldehydes and ketones react with hydride reagents to
O form
b. d. None of the choices
a. Alkanes c. Alcohols
Prepared by: Engr. Quennie Marie L. Bañares
Organic Chemistry 2
b. Alkenes d. Ethers 48. What is the systematic name for the given compound?
40. Carbonyl compounds undergo this type of reaction
a. Electrophilic addition
b. Nucleophilic addition
c. Electrophilic substitution
d. Nucleophilic substitution
a. Methylphenylamine c. N-phenylamine
41. What is the IUPAC name of the compound given below? b. Methylphenylamide d. N-methylamine
49. What would be the product of propanal after
undergoing Clemmensen reduction?
a. Propane c. Propene
a. Ethyl butyrate c. butyl ethanoate
b. Propanoic acid d. Propanone
b. Ethyl butanoate d. butyl ethyl ester
50. Which among the choices is the reactant to complete
42. What is the product when an acyl chloride reacts with
the given reaction?
an alcohol?
a. Ester c. acid anhydride
b. Aldehyde d. carboxylic acid
(1) Na2Cr2O7, H2SO4, H2O
???
43. Which of the following is the expected product for (2) SOCl2
the reaction given?
NH2
+ ???
a. c.
a. c.
N O
b. d.
NH2
b. d.
44. What is the systematic name for the compound given?
a. Ethanoic anhydride
b. 3-carbonyl ester
c. Butanoic ethanoic anhydride
d. Ethanoic propanoic anhydride
45. When acid anhydride reacts with water in pyridine, it
forms which of the following?
a. Acid halides c. Ester
b. No reaction d. Carboxylic acid
46. What is the expected product for the reaction given?
Pyridine
+ HO-CH3 ???
CH3
a.
b. The reagent does not need pyridine; therefore, it
cannot proceed.
c. Both A and D
d.
47. The reaction of a two-carbon alcohol that reacts with
a two-carbon carboxylic acid produces which of the
following?
a. Ethylcarbonyl acid c. Propanoic acid
b. Ethyl acetate d. Propyl acetate
Prepared by: Engr. Quennie Marie L. Bañares
You might also like
- ACS Review 12 Reactions of Arenes - Electrophilic Aromatic SDocument12 pagesACS Review 12 Reactions of Arenes - Electrophilic Aromatic SMohamad HabbabaNo ratings yet
- The Roots of Organic DevelopmentDocument579 pagesThe Roots of Organic DevelopmentFernando MarquesNo ratings yet
- ACS Review 24 PhenolsDocument10 pagesACS Review 24 PhenolsJana BazziNo ratings yet
- Chemistry Paper 1 2009Document7 pagesChemistry Paper 1 2009Robert EdwardsNo ratings yet
- Organic ChemistryDocument36 pagesOrganic ChemistryPepy PeachNo ratings yet
- Organic Chemistry Board Exam Questions PDFDocument10 pagesOrganic Chemistry Board Exam Questions PDFDonPedrewNo ratings yet
- ACS Review 9 AlkynesDocument9 pagesACS Review 9 AlkynesMohamad HabbabaNo ratings yet
- ACS Review 17 Aldehydes and Ketones - Nucleophilic AdditionDocument14 pagesACS Review 17 Aldehydes and Ketones - Nucleophilic AdditionJana Bazzi100% (1)
- Day1 CompiledDocument39 pagesDay1 CompiledWinsletJoyDauagNo ratings yet
- Chemistry of Benzene: Electrophilic Aromatic SubstitutionDocument80 pagesChemistry of Benzene: Electrophilic Aromatic Substitution張湧浩100% (1)
- Aromatic Compounds: © 2006 Thomson Higher EducationDocument103 pagesAromatic Compounds: © 2006 Thomson Higher Educationbrigittanwp putriNo ratings yet
- Organic Reactions v3Document466 pagesOrganic Reactions v3rhozab100% (2)
- 14-Friedel Crafts Acylation FerroceneDocument10 pages14-Friedel Crafts Acylation FerroceneNguyen Minh Duc100% (1)
- Competency Exam in Organic ChemistryDocument4 pagesCompetency Exam in Organic ChemistryRaymond Yabut100% (1)
- Orgchem 2nd TermDocument7 pagesOrgchem 2nd Termsophia del rosarioNo ratings yet
- Organic Chemistry Mock Exam (ANSWER KEY)Document7 pagesOrganic Chemistry Mock Exam (ANSWER KEY)k.talle039No ratings yet
- Organic Chemistry Q&A - 1Document4 pagesOrganic Chemistry Q&A - 1Abaring KathrynaNo ratings yet
- Organic ChemistryDocument2 pagesOrganic ChemistryKim SeungminNo ratings yet
- Chemistry Sma 2: FINAL EXAM SEM-2 2013/2014Document3 pagesChemistry Sma 2: FINAL EXAM SEM-2 2013/2014Arda RahmainiNo ratings yet
- Subject: Chemistry Practice Test-10: Only One Option Is CorrectDocument5 pagesSubject: Chemistry Practice Test-10: Only One Option Is Correctaabha esqueNo ratings yet
- Set 1 PSPM Dk024Document7 pagesSet 1 PSPM Dk024anis fazilaNo ratings yet
- High School 2 Worksheet Question Ans 09-22Document7 pagesHigh School 2 Worksheet Question Ans 09-22Jasmina DezmicNo ratings yet
- Chapter 2 Form5Document9 pagesChapter 2 Form5Zulkifli Bin Pari100% (1)
- 04 Chemistry Unit-10 (Student Copy)Document4 pages04 Chemistry Unit-10 (Student Copy)Kamran ShabbirNo ratings yet
- Review Questions and ProblemsDocument6 pagesReview Questions and Problemsmache dumadNo ratings yet
- CHEM201 FinalExam AnswersDocument16 pagesCHEM201 FinalExam AnswersGlenn Farah Faye RausaNo ratings yet
- ACS Review 23 Aryl HalidesDocument10 pagesACS Review 23 Aryl HalidesmarychakkourNo ratings yet
- Organic-IB-MC-Exam Qu-AnsDocument3 pagesOrganic-IB-MC-Exam Qu-Ansbernardowusubempah123No ratings yet
- Elimination Reactions-McqDocument3 pagesElimination Reactions-Mcqirudayajyothi75% (4)
- Screenshot 2023-09-02 at 9.04.15 PMDocument26 pagesScreenshot 2023-09-02 at 9.04.15 PMleenabed003No ratings yet
- Aromatic Compounds (For IIT)Document2 pagesAromatic Compounds (For IIT)NishthaNo ratings yet
- AlkenesAlkynesExercisesDocument2 pagesAlkenesAlkynesExercisesAR LazagaNo ratings yet
- 1st QUIZ CHEM FINALS PDFDocument5 pages1st QUIZ CHEM FINALS PDFKEZIAH DAWN DABATIANNo ratings yet
- Board Problem TemplateDocument59 pagesBoard Problem TemplategirlwithglassesNo ratings yet
- Aldehyde Ketone Carboxylic Acid pt1Document2 pagesAldehyde Ketone Carboxylic Acid pt1Dharmvir TantyNo ratings yet
- RChE SUMMARY DIAG JAN2024Document16 pagesRChE SUMMARY DIAG JAN2024Paulo Emmanuele BetitaNo ratings yet
- Chem 33 1st LE 2223 SamplexDocument4 pagesChem 33 1st LE 2223 SamplexKayeNo ratings yet
- Organic Chemistry With AnswersDocument3 pagesOrganic Chemistry With AnswersAhmed HashkarNo ratings yet
- Alcohols, Phenols & Ethers Q&ADocument9 pagesAlcohols, Phenols & Ethers Q&AGaurav nayakNo ratings yet
- Question Based On Name ReactionDocument4 pagesQuestion Based On Name ReactionSelcouth elysianNo ratings yet
- Set 2 PSPM Dk024Document7 pagesSet 2 PSPM Dk024anis fazilaNo ratings yet
- Xii - Ch-Amines-QsDocument6 pagesXii - Ch-Amines-Qskaushiksarathi89No ratings yet
- BEC ChemDocument7 pagesBEC ChemSka dooshNo ratings yet
- Chemistry SSC II Paper I-3Document8 pagesChemistry SSC II Paper I-3Muhammad ImranNo ratings yet
- D0597551 CHEM12 C2300 CTBS MigDocument5 pagesD0597551 CHEM12 C2300 CTBS MigHitman KillerNo ratings yet
- Chem 12 Term 1Document5 pagesChem 12 Term 1shikhajha9b33No ratings yet
- Module 4.2 Activity PDFDocument6 pagesModule 4.2 Activity PDFbadajos2317758No ratings yet
- Multiple Choice Questions: Fossil Fuels 1985Document6 pagesMultiple Choice Questions: Fossil Fuels 1985api-3826629No ratings yet
- TaskDocument10 pagesTaskVaaruna RamakrishnanNo ratings yet
- Haloalkane 12th Assigment TDocument2 pagesHaloalkane 12th Assigment TAnanya KanojiaNo ratings yet
- ChemDocument10 pagesChemYnnoNo ratings yet
- Set 4 PSPM Dk024Document7 pagesSet 4 PSPM Dk024anis fazilaNo ratings yet
- 12TH ChemistryDocument11 pages12TH ChemistryAkshatNo ratings yet
- Model Paper-2Document4 pagesModel Paper-2mkrishna collegeNo ratings yet
- HSSC II Chemistry AKU-EB June 2021Document11 pagesHSSC II Chemistry AKU-EB June 2021Shahid Ur RehmanNo ratings yet
- Chemistry McqsDocument10 pagesChemistry McqssabeehNo ratings yet
- PRACTICE MCQ HYDROCARBONS - 11ScADocument7 pagesPRACTICE MCQ HYDROCARBONS - 11ScAArda RahmainiNo ratings yet
- Q (Lab) - Revised - 50 - Filerted - QDocument6 pagesQ (Lab) - Revised - 50 - Filerted - Qbikas_sahaNo ratings yet
- 8 - QP - Aldehyde Ketone Carboxylic AcidDocument8 pages8 - QP - Aldehyde Ketone Carboxylic AcidJems Chaudhary100% (1)
- Govt. Degree Girls College Block K North Nazimabad Section A (M.C.QS) Xii-ChemistryDocument5 pagesGovt. Degree Girls College Block K North Nazimabad Section A (M.C.QS) Xii-ChemistryImran UlhaqueqNo ratings yet
- Haloalkanes & Haloarenes Q & ADocument9 pagesHaloalkanes & Haloarenes Q & AYash JoshiNo ratings yet
- Extra Tutorial FIS 2054 (1-5)Document4 pagesExtra Tutorial FIS 2054 (1-5)Na'im SuhaimiNo ratings yet
- Topic 10 Organic Chemistry 1Document7 pagesTopic 10 Organic Chemistry 1locodeno07No ratings yet
- Progress in Reaction Kinetics: Volume 6From EverandProgress in Reaction Kinetics: Volume 6K. R. JenningsNo ratings yet
- Annual Reports in Organic Synthesis — 1971From EverandAnnual Reports in Organic Synthesis — 1971John McMurryNo ratings yet
- Assignment 4 Reactions of Aromatic Compounds AnswersDocument11 pagesAssignment 4 Reactions of Aromatic Compounds AnswersJonathan Yeung100% (1)
- Friedel Crafts AcylationDocument6 pagesFriedel Crafts AcylationmahdiislamNo ratings yet
- Reduction DisconectionDocument5 pagesReduction DisconectionGabriel FloresNo ratings yet
- 12th Chem. Ch. 9 - Nauman Sadaf - CompressedDocument12 pages12th Chem. Ch. 9 - Nauman Sadaf - Compressedmuzammala099No ratings yet
- Catalytic Properties of Thallium-Containing Mesoporous SilicasDocument5 pagesCatalytic Properties of Thallium-Containing Mesoporous Silicaslucian_lovNo ratings yet
- Content-Based Discussion (Benzene)Document46 pagesContent-Based Discussion (Benzene)nang kubaiNo ratings yet
- Reaction Notes For Organic ChemistryDocument11 pagesReaction Notes For Organic ChemistryTyler Lawrence CoyeNo ratings yet
- Exercise 2Document3 pagesExercise 2KelsierNo ratings yet
- CBSE Class 12 Chemistry Chapter 11 - Alcohols, Phenols and Ethers Important Questions 2023-24Document37 pagesCBSE Class 12 Chemistry Chapter 11 - Alcohols, Phenols and Ethers Important Questions 2023-24Subramanian VasanthiNo ratings yet
- 010F CAcylationPtVIII PDFDocument7 pages010F CAcylationPtVIII PDFMONSE HERNANDEZNo ratings yet
- Electrophilic Aromatic Substitutions Reactions of BenzeneDocument1 pageElectrophilic Aromatic Substitutions Reactions of BenzenepolinaNo ratings yet
- REaction MechanismsDocument5 pagesREaction MechanismstaizokaiNo ratings yet
- Hydrocarbon - Practice SheetDocument3 pagesHydrocarbon - Practice SheetAbhishek PathakNo ratings yet
- Willemse J. A. - Carbon Monoxide As Reagent in The Formulation of Aromatic Compounds (2003)Document137 pagesWillemse J. A. - Carbon Monoxide As Reagent in The Formulation of Aromatic Compounds (2003)Seif SkiNo ratings yet
- CH-150: Basic Organic Chemistry (Sem-I) : Chapter 1-StereochemistryDocument11 pagesCH-150: Basic Organic Chemistry (Sem-I) : Chapter 1-StereochemistryRajesh PatilNo ratings yet
- Revised Organic Compounds Containing NitrogenDocument70 pagesRevised Organic Compounds Containing NitrogenNabiAliNo ratings yet
- 13239639-đã chuyển đổiDocument9 pages13239639-đã chuyển đổiBùi Quang HuyNo ratings yet
- Full Download Book Heterogeneous Catalysis in Sustainable Synthesis Advances in Green and Sustainable Chemistry PDFDocument51 pagesFull Download Book Heterogeneous Catalysis in Sustainable Synthesis Advances in Green and Sustainable Chemistry PDFrichard.lamar761100% (21)
- Oxford University Press - Online Resource Centre - Multiple Choice QuestionsDocument14 pagesOxford University Press - Online Resource Centre - Multiple Choice QuestionsMirza Bilal Mughal50% (2)
- Exercises On Aromatic CompoundsDocument21 pagesExercises On Aromatic CompoundsSeth Andrew Salih100% (1)
- Reactions of Benzene and Its DerivativeDocument46 pagesReactions of Benzene and Its Derivativequyenda08hhaNo ratings yet
- Chemical Reviews Volume 99 Issue 8 1999 (Doi 10.1021/cr980032t) Welton, Thomas - Room-Temperature Ionic Liquids. Solvents For Synthesis and CatalysisDocument14 pagesChemical Reviews Volume 99 Issue 8 1999 (Doi 10.1021/cr980032t) Welton, Thomas - Room-Temperature Ionic Liquids. Solvents For Synthesis and Catalysissushantkadam75No ratings yet
- Ceb1013 - Lecture 8Document70 pagesCeb1013 - Lecture 8api-533764142No ratings yet