Professional Documents
Culture Documents
Synthesis of Magnesium Oxide and Calculation of Percentage Yield
Uploaded by
Jeffrey PiggottOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Synthesis of Magnesium Oxide and Calculation of Percentage Yield
Uploaded by
Jeffrey PiggottCopyright:
Available Formats
Topic Percentage yield Level GCSE (or any course for students
aged 11-16)
Outcomes 1. To carry out an experiment to make MgO and calculate
percentage yield and record results in a suitable table
2. To understand why experiments do not always produce a
percentage yield of 100%
3. To consider the importance of percentage yield to an
industrial chemist
Synthesis of magnesium oxide and calculation of percentage
yield
percentage yield = (actual yield / theoretical yield) x 100
Background: In an ideal world, when you carry out a chemical reaction the
percentage yield would be 100% i.e. all the reactants
would react to produce your product, and none of the
product would be lost during the synthesis. However,
most reactions do not result in a 100% yield. Today we
are going to work out the percentage yield for the
synthesis of magnesium oxide.
Magnesium oxide can be used in the treatment of
heartburn. You are going to imagine that you are a
research chemist working for a pharmaceutical company
that is interested in the reaction below. You are going to
work out the percentage yield of the reaction below to see
if this is a viable way for your company to make magnesium oxide.
Aim: to prepare MgO, calculate the percentage yield and evaluate the method of
preparation.
2 Mg + O2 2 MgO
Before you begin the experiment you will need to produce a results table for
your results. Read the method below and then draw a suitable results table in
this box.
www.thescienceteacher.co.uk | resources for science teachers who like to think
Method:
1. Sand paper the Mg ribbon.
2. Coil the ribbon around a pencil.
3. Weigh an empty crucible and record the mass. Add Mg and reweigh.
Record the new mass. Work out the mass of the Mg used and record
in your table.
4. Set up the apparatus as shown below.
5. Heat the magnesium ribbon and every so often lift the lid using the
tongs to let more air in. DO NOT LOOK DIRECTLY AT THE FLAME.
6. When the reaction is complete let the apparatus cool.
7. Re-weigh the crucible. What is the mass of the magnesium oxide that
was formed?
Complete the table below:
Step in method Why?
Sand paper the Mg ribbon.
Coil the ribbon around a pencil.
Heat the magnesium ribbon and
every so often lift the lid of the
crucible.
www.thescienceteacher.co.uk | resources for science teachers who like to think
Questions:
1. What was the actual yield of MgO made in your experiment?
2. Calculate the percentage yield for your synthesis.
3. Why is the percentage yield not 100%? How could you carry out the
experiment differently to improve your percentage yield?
4. Do you consider this reaction to be a good way for your company to
prepare MgO? Explain with reference to the percentage yield, costs of
reactants and method of preparation whether you would recommend
this method to make MgO on a large scale.
5. One problem with this method is that magnesium nitride is also
formed. How does this affect your percentage yield calculated in
questions 3?
www.thescienceteacher.co.uk | resources for science teachers who like to think
You might also like
- Michael and Aldol Condensation - Organic Lab 39Document5 pagesMichael and Aldol Condensation - Organic Lab 39soccerjake18100% (7)
- Chemistry Notes PT 1Document55 pagesChemistry Notes PT 1EdcademiaNo ratings yet
- Stoichiometric ProblemsDocument2 pagesStoichiometric ProblemsJep Balisi PayusanNo ratings yet
- Pharmaceutical Organic Chemistry I Lab ManualDocument57 pagesPharmaceutical Organic Chemistry I Lab ManualDeep Mali100% (1)
- Chem 242 Manual 2018-2019 FinalDocument79 pagesChem 242 Manual 2018-2019 FinalClara RyuNo ratings yet
- Solutions To Physics I C Vector Worksheet IDocument5 pagesSolutions To Physics I C Vector Worksheet IJeffrey PiggottNo ratings yet
- Solutions To Physics I C Vector Worksheet IDocument5 pagesSolutions To Physics I C Vector Worksheet IJeffrey PiggottNo ratings yet
- Solutions To Physics I C Vector Worksheet IDocument5 pagesSolutions To Physics I C Vector Worksheet IJeffrey PiggottNo ratings yet
- 06 - LectureSlides - As PDFDocument151 pages06 - LectureSlides - As PDFNiaz YousafzaiNo ratings yet
- 06 - LectureSlides - As PDFDocument151 pages06 - LectureSlides - As PDFNiaz YousafzaiNo ratings yet
- The Ultimate ENGAA Guide: Engineering Admissions Assessment preparation resources - 2022 entry, 400+ practice questions and past papers, worked solutions, techniques, score boostingFrom EverandThe Ultimate ENGAA Guide: Engineering Admissions Assessment preparation resources - 2022 entry, 400+ practice questions and past papers, worked solutions, techniques, score boostingNo ratings yet
- Worksheet Chemistry Electrolysis Ks4Document4 pagesWorksheet Chemistry Electrolysis Ks4Jeffrey PiggottNo ratings yet
- Limiting Reagent Practice ProblemsDocument2 pagesLimiting Reagent Practice ProblemsJanaina LeitinhoNo ratings yet
- Introduction to Dynamic Macroeconomic General Equilibrium ModelsFrom EverandIntroduction to Dynamic Macroeconomic General Equilibrium ModelsNo ratings yet
- I. Objectives: Roxette R. RoseteDocument3 pagesI. Objectives: Roxette R. RoseteRoxette RoseteNo ratings yet
- Lab Safety Skills Worksheet 3Document2 pagesLab Safety Skills Worksheet 3Jeffrey PiggottNo ratings yet
- Experiment 4: Limiting Reactant, Excess Reactant & Percent YieldDocument43 pagesExperiment 4: Limiting Reactant, Excess Reactant & Percent YieldAfrina FazrulNo ratings yet
- TLE HEG8 ModuleDocument65 pagesTLE HEG8 Modulejoan niniNo ratings yet
- Cambridge International AS and A Level Chemistry (9701) : Practical Booklet 1Document11 pagesCambridge International AS and A Level Chemistry (9701) : Practical Booklet 1Suhashie ThalgaspitiyaNo ratings yet
- Science Grade 10: Department of EducationDocument17 pagesScience Grade 10: Department of EducationLeiNo ratings yet
- Biofuels: Alternative Feedstocks and Conversion ProcessesFrom EverandBiofuels: Alternative Feedstocks and Conversion ProcessesRating: 3 out of 5 stars3/5 (1)
- Magnesium Oxide Lab StoichiometryDocument2 pagesMagnesium Oxide Lab StoichiometryAnh Tuan LeeNo ratings yet
- 05 06 Lab ReportDocument3 pages05 06 Lab ReportMaximilian WuelfingNo ratings yet
- Worksheet 05 07Document3 pagesWorksheet 05 07Kobi ThomasNo ratings yet
- Grade 9, Notes and Assignment No 9Document8 pagesGrade 9, Notes and Assignment No 9madhuri pawarNo ratings yet
- Experiment 5 Reaction of Magnesium With Hydrochloric Acid: OutcomesDocument4 pagesExperiment 5 Reaction of Magnesium With Hydrochloric Acid: OutcomesHarneetNo ratings yet
- MagnesiumOxide LabDocument3 pagesMagnesiumOxide Labwanderlust23100% (1)
- Empirical Formula of Magnesium OxideDocument8 pagesEmpirical Formula of Magnesium OxideAnh Tuan LeeNo ratings yet
- Percent Yield Homework AnswersDocument4 pagesPercent Yield Homework Answerscjbejq76100% (2)
- Chemical Reactions (5E)Document9 pagesChemical Reactions (5E)Olga Becky AlfaroNo ratings yet
- Are You A Practicing Scientist or Engineer?Document9 pagesAre You A Practicing Scientist or Engineer?praba_karanc4916No ratings yet
- Percent Yield: Chemfile Mini-Guide To Problem SolvingDocument11 pagesPercent Yield: Chemfile Mini-Guide To Problem SolvingdhavaleshNo ratings yet
- Limiting Reactants and Product YieldDocument9 pagesLimiting Reactants and Product YieldIan Ochea100% (1)
- Lab 16 - Law of Definite CompositionDocument6 pagesLab 16 - Law of Definite CompositionMicah YapNo ratings yet
- Law of Definite Proportions Lab ReportDocument3 pagesLaw of Definite Proportions Lab Reportrinkeanmark50% (2)
- Burning Magnesium LabDocument2 pagesBurning Magnesium LabRohan MorarNo ratings yet
- LE 005 007 General Chemistry 1 Continuation .Updated FinalDocument26 pagesLE 005 007 General Chemistry 1 Continuation .Updated FinalShaman KingNo ratings yet
- Percent Yield and Theoretical Yield ConceptsDocument17 pagesPercent Yield and Theoretical Yield ConceptsRoger Wyvern Hakdogen WiseNo ratings yet
- Nitration of Methyl BenzoateDocument6 pagesNitration of Methyl BenzoateHidayu AdnanNo ratings yet
- Determining the empirical formula of magnesium oxide experimentDocument2 pagesDetermining the empirical formula of magnesium oxide experimentClaudia JaukinNo ratings yet
- Limiting Reactants and YieldDocument20 pagesLimiting Reactants and YieldFaadilahJacobsNo ratings yet
- Determination of The Empirical Formula of MgODocument7 pagesDetermination of The Empirical Formula of MgOCicy IrnaNo ratings yet
- SHS Gen - Chem 1-Q1 MEL-12 Week-3Document10 pagesSHS Gen - Chem 1-Q1 MEL-12 Week-3thatkidmarco22No ratings yet
- HighGrade's nutrient formula enhances growth with Pro-Tekt and Epsom SaltsDocument9 pagesHighGrade's nutrient formula enhances growth with Pro-Tekt and Epsom SaltsLisuNo ratings yet
- Basic Chemistry Modul 2022 1Document47 pagesBasic Chemistry Modul 2022 1Ohh ChimmyNo ratings yet
- Lab 9 120MP Testing Means in Minitab: Example 1Document2 pagesLab 9 120MP Testing Means in Minitab: Example 1LexNo ratings yet
- Smore StoichiometryDocument4 pagesSmore Stoichiometry임민수No ratings yet
- MgO LabDocument1 pageMgO Labapi-3697114No ratings yet
- Manual Biochemistry-10-2017Document65 pagesManual Biochemistry-10-2017Dental LecturesMMQNo ratings yet
- 123 283276 250386 The Ideal Gas Equation PresentationDocument28 pages123 283276 250386 The Ideal Gas Equation PresentationhdawgNo ratings yet
- % Comp With OreoDocument2 pages% Comp With OreoRYAN SCOTTNo ratings yet
- Lab Simulation Factors Affecting Enzyme ReactionsDocument15 pagesLab Simulation Factors Affecting Enzyme ReactionsJuan Manuel Herrera NavasNo ratings yet
- Magnesium Oxide Empirical FormulaDocument5 pagesMagnesium Oxide Empirical FormulaKartz EswarNo ratings yet
- Econ 100 Midterm practice_KEYDocument7 pagesEcon 100 Midterm practice_KEYIzza NaseerNo ratings yet
- Equilibrium Lab: Gizmo Simulations for Chemical EquilibriumDocument11 pagesEquilibrium Lab: Gizmo Simulations for Chemical EquilibriumGabriela PopaNo ratings yet
- How Does The Amount of Magnesium Affect The Amount of Gas Produced in One MinuteDocument6 pagesHow Does The Amount of Magnesium Affect The Amount of Gas Produced in One MinuteChalee Lee100% (1)
- 1.2 3 Empirical Formula CompoundDocument6 pages1.2 3 Empirical Formula CompoundTrương Quốc HuyNo ratings yet
- 3 Lab Manual For Gen Chem1Document39 pages3 Lab Manual For Gen Chem1Wei WeiNo ratings yet
- (Q1) MODULE 7 - Percentage Yield PDFDocument16 pages(Q1) MODULE 7 - Percentage Yield PDFJewel SantiagoNo ratings yet
- Percent Yield: Group 5Document21 pagesPercent Yield: Group 5kobe caneteNo ratings yet
- Problems in The Quality of ProductsDocument2 pagesProblems in The Quality of ProductsEngr Arfan Ali DhamrahoNo ratings yet
- Airbag Stoichiometry LabDocument4 pagesAirbag Stoichiometry LabJoanne Moh0% (1)
- PhotosynthesisLabSE Elle KDocument8 pagesPhotosynthesisLabSE Elle KmarieNo ratings yet
- Sorsogon National High School: Self-Directed Learning Activity Sheet in General Chemistry 1 (Module 6)Document4 pagesSorsogon National High School: Self-Directed Learning Activity Sheet in General Chemistry 1 (Module 6)Jorgia lianne UrbanoNo ratings yet
- Stoichiometry II Homework WorksheetDocument8 pagesStoichiometry II Homework Worksheetafnojbsgnxzaed100% (1)
- DoE AnwarDocument23 pagesDoE AnwarRayandrea PratamaNo ratings yet
- Calculation Workbook 5Document50 pagesCalculation Workbook 5Brenda JacksonNo ratings yet
- A Level Chemistry Balancing Equations: Instructions and Answers For TeachersDocument6 pagesA Level Chemistry Balancing Equations: Instructions and Answers For TeachersIgnacio Jr. PaguyoNo ratings yet
- Unit 7 Test: Name: - Date: - PeriodDocument2 pagesUnit 7 Test: Name: - Date: - PeriodmamazookeeprNo ratings yet
- Department of Chemistry Chemistry 211 Inorganic Chemistry: University of The Western CapeDocument23 pagesDepartment of Chemistry Chemistry 211 Inorganic Chemistry: University of The Western CapeCozzy 808No ratings yet
- Michael Erwin YantoDocument2 pagesMichael Erwin Yantomicks4522No ratings yet
- Student Exploration: Photosynthesis Lab: OxygenDocument7 pagesStudent Exploration: Photosynthesis Lab: OxygenAnthony HernandezNo ratings yet
- 9702 s11 QP 41 Question On SHMDocument2 pages9702 s11 QP 41 Question On SHMJeffrey PiggottNo ratings yet
- Kinema TicsDocument63 pagesKinema TicsJeffrey PiggottNo ratings yet
- 9702 s11 QP 41 Question On SHMDocument2 pages9702 s11 QP 41 Question On SHMJeffrey PiggottNo ratings yet
- IGCSE Science Homework #1 - Lab Safety: View The Picture Below and Answer The Questions On The Next PageDocument2 pagesIGCSE Science Homework #1 - Lab Safety: View The Picture Below and Answer The Questions On The Next PageJeffrey PiggottNo ratings yet
- Answer Key Electrolytic Cell WorksheetDocument2 pagesAnswer Key Electrolytic Cell WorksheetJeffrey PiggottNo ratings yet
- Chemistry WorkBook For Dummies (PDFDrive) - Pages-31-35Document5 pagesChemistry WorkBook For Dummies (PDFDrive) - Pages-31-35Jeffrey PiggottNo ratings yet
- 9702 - w20 - QP - 11 - NotesDocument20 pages9702 - w20 - QP - 11 - NotesPham Thanh MinhNo ratings yet
- Electrolysis TDocument8 pagesElectrolysis TJeffrey PiggottNo ratings yet
- Vernier Caliper 9Document3 pagesVernier Caliper 9Jeffrey PiggottNo ratings yet
- Forces, Movement, Shape and Momentum 1 QPDocument14 pagesForces, Movement, Shape and Momentum 1 QPJeffrey PiggottNo ratings yet
- Vernier Caliper Worksheet +: Q1. What Is The Reading On The Vernier Scales Below? The Scale Is in Metric UnitsDocument3 pagesVernier Caliper Worksheet +: Q1. What Is The Reading On The Vernier Scales Below? The Scale Is in Metric UnitsJeffrey PiggottNo ratings yet
- Chemistry WorkBook For Dummies (PDFDrive) - Pages-31-35Document5 pagesChemistry WorkBook For Dummies (PDFDrive) - Pages-31-35Jeffrey PiggottNo ratings yet
- Graph and acceleration analysis of falling objectDocument6 pagesGraph and acceleration analysis of falling objectJeffrey PiggottNo ratings yet
- Paperless Productivity With SmallpdfDocument22 pagesPaperless Productivity With SmallpdfJeffrey Piggott100% (1)
- 16 Pdfsam 2 Int QualitativeDocument1 page16 Pdfsam 2 Int QualitativeJeffrey PiggottNo ratings yet
- Graph and acceleration analysis of falling objectDocument6 pagesGraph and acceleration analysis of falling objectJeffrey PiggottNo ratings yet
- Kinematics Vocabulary Guide for Units, Vectors & FormulasDocument17 pagesKinematics Vocabulary Guide for Units, Vectors & FormulasJeffrey PiggottNo ratings yet
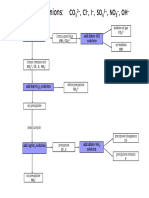
- Identifying Cations FlowchartDocument1 pageIdentifying Cations FlowchartJeffrey PiggottNo ratings yet
- Identifying Anions FlowchartDocument1 pageIdentifying Anions FlowchartJeffrey PiggottNo ratings yet
- Space Scientists InvestigationDocument1 pageSpace Scientists InvestigationJeffrey PiggottNo ratings yet
- 16 Pdfsam 2 Int QualitativeDocument1 page16 Pdfsam 2 Int QualitativeJeffrey PiggottNo ratings yet
- T1 Exp - Density of Microscope SlideDocument1 pageT1 Exp - Density of Microscope SlideJeffrey PiggottNo ratings yet
- Space Scientists InvestigationDocument1 pageSpace Scientists InvestigationJeffrey PiggottNo ratings yet
- Effluent Proteins From Rapeseed-Cheese Whey Protein Coprecipitation ProcessDocument6 pagesEffluent Proteins From Rapeseed-Cheese Whey Protein Coprecipitation ProcesskaltoumNo ratings yet
- β-Keto EstersDocument4 pagesβ-Keto EstersmarianaNo ratings yet
- Reviewer ScienceDocument4 pagesReviewer ScienceJacqueline SuarezNo ratings yet
- B.Sc. Engineering Exam Covers Chemical Engineering ConceptsDocument2 pagesB.Sc. Engineering Exam Covers Chemical Engineering ConceptsMuhaiminul Hasan EmonNo ratings yet
- CH 3 StoichiometryDocument30 pagesCH 3 StoichiometryGhina Nur FadhilahNo ratings yet
- Introductory Chemistry 5th Edition Ebook PDFDocument41 pagesIntroductory Chemistry 5th Edition Ebook PDFmarleen.may408100% (36)
- C16 - Lec 07 - Chemical ReactionsDocument62 pagesC16 - Lec 07 - Chemical ReactionsJohn Lloyd GildoNo ratings yet
- Chem 1104Document41 pagesChem 1104Paul Jhon EugenioNo ratings yet
- Apprentice Teaching: Lesson Plan Summary Percent Yield & Limiting ReagentsDocument3 pagesApprentice Teaching: Lesson Plan Summary Percent Yield & Limiting Reagentsapi-533864204No ratings yet
- Modelling The Kinetics of Biolubricant From Castor Oil Using Novozym in A Fluidized Bed ReactorDocument8 pagesModelling The Kinetics of Biolubricant From Castor Oil Using Novozym in A Fluidized Bed ReactorBreak LimsNo ratings yet
- Synt432 PrepCuA4H2ODocument12 pagesSynt432 PrepCuA4H2OWisi Wasi100% (1)
- Gen Chem NotesDocument13 pagesGen Chem NotessusontangofeliptianjayNo ratings yet
- Topic Test QP G11 (Quantitative Aspects of Chemical Change 2023) (F)Document6 pagesTopic Test QP G11 (Quantitative Aspects of Chemical Change 2023) (F)Karlos 03No ratings yet
- BTEC Level 3 Unit 1 Past Paper Questions: CalculationsDocument9 pagesBTEC Level 3 Unit 1 Past Paper Questions: CalculationsabdiNo ratings yet
- Chemistry+1+Tutor+ +vol+2+ +worksheet+12+ +Percent+Yield+in+Chemical+ReactionsDocument11 pagesChemistry+1+Tutor+ +vol+2+ +worksheet+12+ +Percent+Yield+in+Chemical+ReactionsCCSC124-Soham MaityNo ratings yet
- YieldDocument11 pagesYieldNaeem AshrafNo ratings yet
- Chem Int CC CH 12 - Stoichiometry - Answers (09.15)Document7 pagesChem Int CC CH 12 - Stoichiometry - Answers (09.15)Emma GillesNo ratings yet
- Preparation of AcetanilideDocument10 pagesPreparation of AcetanilideJaymarine SpenceNo ratings yet
- Objectives: FIGURE A: Example of Coordination CompoundsDocument7 pagesObjectives: FIGURE A: Example of Coordination CompoundsNurul izzatiNo ratings yet
- 3361L Syllabus FA12Document3 pages3361L Syllabus FA12Andre VilliersNo ratings yet
- UCTM2017Document13 pagesUCTM2017Medha HebbarNo ratings yet
- Part II - Nederlandse Cocaine Fabriek - NCFDocument222 pagesPart II - Nederlandse Cocaine Fabriek - NCFscribdcrawler100% (1)
- Oxidation of Cyclohexane and Ethylbenzene by Hydrogen Peroxide Over Co-Substituted Heteropolytungstate CatalystDocument6 pagesOxidation of Cyclohexane and Ethylbenzene by Hydrogen Peroxide Over Co-Substituted Heteropolytungstate Catalystrungrawin ngamkhumNo ratings yet