0% found this document useful (0 votes)
889 views12 pagesUnit 3 - The Chemistry of Engineering Materials Polymers
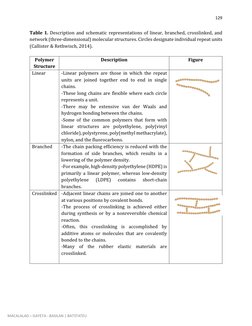
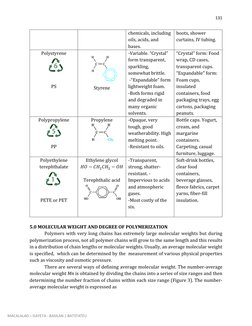
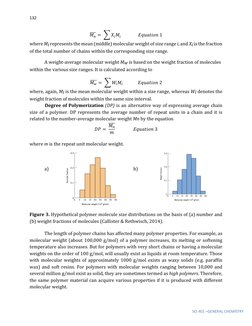
This document discusses the chemistry and properties of polymer materials. It begins by defining polymers as large molecular compounds made up of repeating monomer units. The document then discusses the molecular structure of common polymers like polyethylene, polytetrafluoroethylene, and poly(vinyl chloride). It explains how these polymers are formed through addition polymerization reactions between monomer units. The document also describes different types of polymer molecular structures including linear, branched, crosslinked, and network structures. It provides schematic representations of these different structural arrangements.
Uploaded by
Niña Viaña BinayCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
889 views12 pagesUnit 3 - The Chemistry of Engineering Materials Polymers
This document discusses the chemistry and properties of polymer materials. It begins by defining polymers as large molecular compounds made up of repeating monomer units. The document then discusses the molecular structure of common polymers like polyethylene, polytetrafluoroethylene, and poly(vinyl chloride). It explains how these polymers are formed through addition polymerization reactions between monomer units. The document also describes different types of polymer molecular structures including linear, branched, crosslinked, and network structures. It provides schematic representations of these different structural arrangements.
Uploaded by
Niña Viaña BinayCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd