Professional Documents
Culture Documents
Lectura Complementaria. Método de Referencia para El Recuento de Plaquetas
Uploaded by
Flor GianoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lectura Complementaria. Método de Referencia para El Recuento de Plaquetas
Uploaded by
Flor GianoCopyright:
Available Formats
Coagulation and Transfusion Medicine / REFERENCE METHOD FOR PLATELET COUNTING
Platelet Counting by the RBC/Platelet Ratio Method
A Reference Method
International Council for Standardization in Haematology Expert Panel on Cytometry* and
International Society of Laboratory Hematology Task Force on Platelet Counting†
Key Words: Flow cytometry; Platelets; Platelet count; Reference method; Value assignment; Whole blood calibration
Abstract The International Council for Standardization in Haema-
The International Council for Standardization in tology (ICSH) has recommended reference methods for the
Haematology (ICSH) and the International Society of determination of hemoglobin,1 packed RBC volume,2 and the
Laboratory Hematology (ISLH) recommend the enumeration of erythrocytes and leukocytes.3 Thus, there
counting of specifically labeled platelets relative to the remains the need for a reference method for the enumeration
RBCs with a fluorescence flow cytometer, together with of platelets to complete the set of methods required for (whole
an accurate RBC count determined with a blood) calibration of the traditional CBC count and for assign-
semiautomated, single-channel aperture-impedance ment of values to hematology analyzer calibration materials.
counter as a reference method for the enumeration of The ICSH has defined a reference method as “a clearly
platelets. Fresh EDTA-anticoagulated venous blood and exactly described technique for a particular determina-
specimens are measured within 4 hours of the draw. tion which, in the opinion of a defined authority, provides
The specimen is prediluted (1:20) and the platelets sufficiently accurate and precise laboratory data for it to be
labeled with two monoclonal antibodies specific to a used to assess the validity of other laboratory methods for
cluster of differentiation common to all platelets. A final this determination. The accuracy of the reference method
1:1,000 dilution is made and at least 50,000 events with must be established by comparison with a definitive method
a minimum of 1,000 platelet events are counted with a where one exists, and the degree of inaccuracy and impreci-
flow cytometer to determine the RBC/platelet ratio. The sion must be stated.”
platelet count is then calculated from this ratio and the Lacking a true reference method for the platelet count,
RBC concentration of the original blood specimen. the ICSH Expert Panel on Cytometry previously has recom-
mended selected methods for assigning platelet count values
to fresh blood used for whole blood calibration of hematology
analyzers.4 These selected methods include hemocytometer
phase contrast microscopy with an ammonium oxalate
diluent5 and the determination of the RBC/platelet (PLT) ratio
measured with an aperture-impedance counter with a hydro-
dynamic focused flow stream (indirect platelet counting).
Obtaining accurate, precise, and reliable platelet counts
as a reference for the calibration of hematology analyzers has
been a continuing problem for 2 reasons: (1) difficulties
discriminating the small platelet signals from those of debris
and spurious noise, and (2) the elimination of interference
with the platelet count by substantially more numerous
erythrocytes.
460 Am J Clin Pathol 2001;115:460-464 © American Society of Clinical Pathologists
Coagulation and Transfusion Medicine / ORIGINAL ARTICLE
With the increasing use of fluorescent flow cytometry, anticoagulated with EDTA, dipotassium salt, or tripotassium
there has been renewed interest in indirect platelet counting, salt, 3.7 to 5.4 µmol/mL of blood (K2EDTA, anhydrous,
ie, the determination of the RBC/PLT ratio. These indirect Chemical Abstracts Service [CAS] 25102-12-9, formula
counting methods are performed on a flow cytometer after the weight of 368.4, 1.4-2.0 mg/mL; K3EDTA, dihydrate, CAS
platelets have been labeled fluorescently with a monoclonal 6550-24-8, formula weight of 442.5, 1.6-2.4 mg/mL). Only
antibody specific to a cluster of differentiation common to all K2EDTA should be used when the same specimens also are
(resting and activated) platelets. A number of such antibodies required for hematocrit and mean corpuscular volume
have been described, including anti-CD42a6-8; anti-CD41b8; measurements.12 The containers must have sufficient air
anti-CD619; and anti-CD41, anti-CD42, and anti-CD61.10,11 remaining to allow for proper mixing. The specimen should
Studies have shown that fluorescent labeling with any one of a be rejected if there is visual hemolysis or if microclots are
number of commercially available monoclonal antibodies present. Maintain the specimens at room temperature (18 C-
seems to be specific and is claimed to be essentially universal, 22 C), and process within 4 hours of obtaining the specimen.
ie, all platelets are labeled in a large variety of blood speci- Do not place specimens on a rocking or other “mixing”
mens.9,11 The preparation and counting with modern flow device. To ensure a homogeneous distribution of RBCs and
cytometers is practical, with good separation between the cell platelets, thoroughly mix the specimen by at least 8 gentle,
types for discrimination purposes. However, RBC-RBC, complete inversions before predilution and antibody
RBC-platelet, and WBC-platelet coincidences remain a labeling.13
problem for cell counting by flow cytometry, since the sensing
zone generally is defined only by the Gaussian distribution of
the laser light and is typically greater than 1,000 µm. Because Glassware
the sensing volume is defined poorly, coincidence correction Care must be taken to avoid the adherence of platelets to
generally is precluded, and serial dilutions must be used to any surface of any container used in storing the original
verify the absence of errors due to coincidence. specimen or any diluted sample. Therefore, plain glass
surfaces must be avoided, and polypropylene or polystyrene
must be used throughout specimen processing.
Recommendation
The ICSH now recommends that an indirect platelet
count, ie, the counting of specifically labeled platelets rela- Instrument Specifications
tive to the RBCs with a fluorescence flow cytometer, For platelet and RBC enumeration, a fluorescent flow
together with an accurate RBC count determined with a cytometer with hydrodynamic focusing and the capability of
semiautomated, single-channel, aperture-impedance particle measuring forward light scatter and fluorescence is used. The
counter, provides a method sufficiently accurate and precise instrument should have sufficient sensitivity to scattered and
to be used for whole blood calibration of cell counters and fluorescein fluorescent light to reliably count fluorescein
for assigning values to calibration materials. isothiocyanate–labeled spherical particles of 2 µm in diameter.
For the whole blood RBC count, a semiautomated,
single-channel, aperture-impedance particle counter is used.
Principle of the Method The instrument should have an orifice diameter of 80 to 100
An EDTA-anticoagulated blood specimen is prediluted µm and a length 70% to 100% of the diameter, and the
in a sterile buffered solution. The platelets then are stained volume displaced during the counting period must be known
with specific fluorescent antibodies. The stained platelets in to within an accuracy of 1%, traceable to a national or inter-
solution are diluted to the counting concentration, and the national metrologic standard.3 (NOTE: At present, no optical
platelets and RBCs are counted on a flow cytometer with particle counters are available that meet the requirement of
thresholds set to discriminate platelets from RBCs on the an accurately known and traceable volume displacement.)
basis of fluorescence amplitude and scatter amplitude. The
RBC/PLT ratio is determined, and the platelet count is calcu-
lated from an accurate RBC count of the sample, obtained Reagents
using a cell counter that meets previous ICSH specifications.3
Diluent
The diluent is phosphate-buffered saline (PBS), 0.01
Blood Specimens mol/L, pH 7.2 to 7.4 with 0.1% bovine serum albumin
Fresh venous blood specimens are collected from appar- (BSA). Steps are as follows:
ently healthy donors by syringe or evacuated container and 1. Dissolve 1.15 g dibasic, anhydrous sodium phosphate
© American Society of Clinical Pathologists Am J Clin Pathol 2001;115:460-464 461
ICSH and ISLH / REFERENCE METHOD FOR PLATELET COUNTING
(Na2HPO4; CAS, 7558-79-4; formula weight, 142.0) in gentle inversions of the specimen tube) blood specimen into
approximately 750 mL of deionized or distilled water. 100 µL of the filtered PBS-BSA diluent.
2. Add 210 mg monobasic, anhydrous potassium phos- 2. Add 5 µL of the anti-CD41 and 5 µL of the anti-
phate (KH2PO4; CAS, 7778-77-0; formula weight, 136.1), CD61 staining solution and incubate for 15 minutes, in the
8.0 g sodium chloride (NaCl; CAS, 7647-14-5; formula dark, at ambient temperature (18 C-22 C).
weight, 58.44), 200 mg potassium chloride (KCl; CAS, NOTE: Consistent results have been obtained when the
7447-40-7; formula weight, 74.55), and 1.0 mL BSA, frac- blood and the anti-CD41 and anti-CD61 staining solutions
tion V. Dilute to 1,000 mL with deionized or distilled water. are pipetted as separate beads in the bottom of the reaction
Store at 4 C to 8 C. tube and the PBS-BSA diluent is added.13
3. Filter the diluent through a low-binding, low-release 3. After 15 minutes, prepare a final 1:1,000 dilution for
membrane filter, with a mean pore diameter of 0.20 to 0.25 counting by adding 4.85 mL of the PBS-BSA diluent. Mix
µm before use. (Good results have been obtained with, eg, well by gentle inversions to ensure proper and equal distribu-
Millex-GV filters, Millipore Intertech, Bradford, MA.13) tion of RBCs and platelets.
4. With the flow cytometer, count a minimum of 50,000
Staining Solution events with a minimum of 1,000 platelet events. The flow
For the staining solution, directly conjugated, fluores- cytometer settings must ensure an acquisition rate of less
cein isothiocyanate–labeled, antibodies against 2 distinct than 3,000 events per second. Events that are positive for
epitopes on the glycoprotein IIb/IIIa complex of platelets are RBC scatter signal, as well as for platelet fluorescence, are
used: antibodies to CD41 and CD61. considered RBC-platelet coincidence events and are added to
NOTE: In a series of 65 blood specimens with platelet both the RBC and the platelet events.
counts ranging from 15 to 900 ·103/µL (15-900 ·109/L), no Either quadrant or bitmap analysis is acceptable, but
difference in labeling was found in 17 specimens; in 25 spec- bitmap analysis is recommended. The use of positive
imens, more platelets were stained by anti-CD41 than by displacement pipettes is recommended.
anti-CD61; and in 23 specimens, more were stained by anti-
CD61 than by anti-CD41 ❚Table 1❚. The reference method Determination of RBC Concentration
thus requires a double-staining technique. Following the ICSH reference method for enumeration
Laboratories must verify that a specific clone or batch of of erythrocytes (and leukocytes), 3 determine the RBC
antibodies results in adequate fluorescent staining of the concentration of the original blood specimen using a semiau-
platelets. The antibody (antibodies) must give a high enough tomated, single-channel, aperture-impedance particle
fluorescent signal of the entire platelet population so that it is counter.
completely resolved from noise and debris and the RBCs on
a plot of log FL1 (fluorescence at 528 nm) vs log FS
(forward scatter). (This usually was greater than the first log
Platelet Count Determination
decade when the background fluorescence was set with a
matched isotype control.) From the flow cytometry data, determine the RBC/PLT
ratio, R, to at least 3 decimal places by using the following
formula:
Procedure R = RBC Events/Platelet Events
Divide the RBC count determined in the original spec-
Enumeration of RBCs and Platelets imen by this ratio, R, to arrive at the platelet count.
Steps are as follows: For example:
1. Pipette 5 µL of the well-mixed (at least 8 complete, RBC Count, 5.44 ·106/µL (5.44 ·1012/L); R = 20.4896
❚Table 1❚
Staining Results
Difference (%)
Staining by Anti-CD41 vs Anti-CD61 No. of Samples Mean SD Total Range
No difference (± 1%) 17 0 — —
Anti-CD41 greater than anti-CD61 (> 1%) 25 4.8 3.04 1.1-12.2
Anti-CD41 less than anti-CD61 (< –1%) 23 4.8 4.62 1.02-19.9
462 Am J Clin Pathol 2001;115:460-464 © American Society of Clinical Pathologists
Coagulation and Transfusion Medicine / ORIGINAL ARTICLE
Platelet Count = 5.44 ·106/µL (5.44 ·1012/L) Divided equation can be simplified and expressed as a function of the
by 20.4896 = 265.5 ·103/µL (265.5 ·109/L) RBC/PLT ratio:
NOTE: In a series of 357 apparently healthy, racially CV2 = (R + 1)/Ntotal
mixed, male and female volunteers, median age 26 years, a and
mean ± SD ratio of 21.572 ± 5.134 was found. CV (%) = [ (R + 1)/Ntotal] ·100
The mean of normal values for the RBC/PLT ratio is
around 20. At this value and with an Ntotal of 50,000, the
statistical imprecision is 2%. Practical experience has
Coincidence
demonstrated the replicate precision for the method to be
Once the assay has been optimally set up, application of about 1.7% and 3% at platelet counts of 280 and 40 ·103/µL
a coincidence correction is not required. However, applica- (280 and 40 ·109/L), respectively.
tion of the coincidence correction equations given in
Appendix 113 is recommended during setup of the assay to
ensure optimal conditions (eg, dilution, flow, or acquisition
Sources of Interference
rate) are achieved and to monitor potential problems with
some pathologic specimens (eg, very high platelet counts). Although labeling with the monoclonal antibodies used
in this method has been shown to be effective with a wide
variety of specimens,12 there exists the potential for spec-
imen-specific interference. Interference could result from an
Imprecision
absence of the binding sites or blocking of the staining reac-
As in all cell counting methods, a fundamental limita- tion in certain specimens. A summary of potential interfer-
tion on the reproducibility of the result is provided by the ences is listed in ❚Table 2❚. Thus, it is important to ensure
statistical variation in the number of cells sampled: the coef- that whole blood specimens used for the calibration of hema-
ficient of variation (CV) is the square root of the reciprocal tology analyzers do not exhibit any of the conditions listed.
of the number of cells counted. In the reference method
described, a proportion, R, is derived from the ratio of the
two counts (number of platelets, NPlt, and number of RBCs,
Evaluation and Transferability
N RBC ) after a fixed number, N, of total cells has been
analyzed. The method, staining with anti-CD41 and anti-CD61 in
The theory of propagation of errors indicates that the parallel, has been tested in 11 laboratories (in France, Japan,
CV of a ratio is given by the variation of the individual the United Kingdom, and the United States); staining with a
measurements added in quadrature: combination of anti-CD41 and anti-CD61 has been tested in
CV2 = (1/NPlt) + (1/NRBC) 4 laboratories (in the United Kingdom and the United
Owing to the relatively large number of RBCs (NRBC = States). Each laboratory analyzed stabilized material and at
approximately Ntotal) and substituting (NRBC/R) for NPlt, the least 60 patient specimens with a range of platelet counts.
❚Table 2❚
Potential Sources of Interference With Platelet Counts in Selected Conditions*
Error Source Effect Condition Frequency
High platelet count Unstable count Thrombocythemia; thrombocytosis Occasional
(platelet activation)
Autoantibodies Blocking of binding site Idiopathic thrombocytopenic purpura Rare
Congenital platelet disorders Lack of binding site Glanzmann thrombasthenia Very rare
glycoprotein IIb/IIIa
Platelet aggregation Platelet clumps Aged blood; EDTA effect Occasional
Platelet-WBC adherence Platelets outside gate Following cardiac bypass; EDTA Occasional
Cold agglutinins RBC clumps Agglutinin-related disorder Occasional
Fragmented RBCs Incorrect RBC count Hemolytic anemia; disseminated intravascular coagulation; Rare
HUS; artificial heart valve
Fragmented WBCs (including False platelet count Leukemia, especially chronic lymphocytic; chemotherapy Occasional
apoptotic fragments)
Abnormal platelet size Platelets outside gate Thrombocytopenia; May-Hegglin anomaly Occasional
EDTA, ethylenediaminetetraacetic acid; HUS, hemolytic uremic syndrome.
* Adapted from Groner W. Draft ICSH reference method for obtaining the ratio of the RBC count to platelet count. ICSH Expert Panel on Cytometry, internal document.
© American Society of Clinical Pathologists Am J Clin Pathol 2001;115:460-464 463
ICSH and ISLH / REFERENCE METHOD FOR PLATELET COUNTING
All laboratories demonstrated excellent intra-assay and 6. Groner W, Mayer K, Chapman ES. An indirect platelet count
acceptable interlaboratory precision. The overall correlations using a platelet-specific monoclonal antibody and flow
cytometry can produce reliable platelet counts for assessing
between anti-CD41 and anti-CD61 and each with anti-CD41 thrombocytopenia. Blood. 1994;84(suppl 1):687a.
and anti-CD61 dual labeling was excellent with no apparent 7. Tanaka C, Ishii T, Fujimoto K. Flow cytometric platelet
bias. The method, using a calibrated automated hematology enumeration utilizing monoclonal antibody CD42a. Clin Lab
analyzer instead of a reference particle counter to determine Haematol. 1996;18:265-269.
the number of RBCs, also has been shown to be a valuable 8. Dickerhof R, von Ruecker A. Enumeration of platelets by
multiparameter flow cytometry using platelet specific
tool for determining the accuracy of platelet counting in antibodies and fluorescent reference particles. Clin Lab
thrombocytopenia.13 Haematol. 1995;17:163-172.
9. Harrison P, Horton A, Grant D, et al. Immunoplatelet
Address reprint requests to Dr Rowan: Executive Secretary ICSH, counting: a proposed new reference procedure. Br J Haematol.
13 Buchanan St, MILNGAVIE, Glasgow G62 8AW, Scotland, UK. 2000;108:228-235.
* Chair: George Klee, MD, Rochester, MN; Secretary: 10. Ault K. Platelet counting: is there room for improvement? Lab
Haematol. 1996;2:139-143.
Guiseppe d’Onofrio, Rome, Italy; and Members: Onno W. van
11. Davis RH, Bigelow NC. Evaluation of flow cytometric
Assendelft, MD, PhD, Atlanta, GA; Brian Bull, MD, Loma Linda, indirect immunoplatelet counting as a reference method for
CA; Ahnond Bunyaratvej, MD, Bangkok, Thailand; M. Buttarello, platelet count calibration. Lab Haematol. 1999;5:15-21.
MD, Padova, Italy; George Colella, PhD, Tarrytown, NY; Bruce
12. International Council for Standardization in Haematology
Davis, MD, South Portland, ME; Keiji Fujimoto, MEng, Kobe, Expert Panel on Cytometry. Recommendations of the
Japan; Warren Groner, Great Neck, NY; Berend Houwen, MD, International Council for Standardization in Haematology for
PhD, Loma Linda, CA; Luc van Hove, MD, PhD, Santa Clara, ethylenediaminetetraacetic acid anticoagulation of blood for
CA; John A. Koepke, MD, Durham, NC; Mitchell Lewis, MD, blood cell counting and sizing. Am J Clin Pathol.
London, England; Sam Machin, MD, London, England; Robert 1993;100:371-372.
Raynor, PhD, Miami, FL; Martin Rowan, MD, Glasgow, 13. Harrison P, Ault KA, Chapman S, et al. An interlaboratory
Scotland; and Noriyuki Tatsumi, MD, Osaka, Japan. study of a candidate reference method for platelet counting.
† Chair: Onno W. van Assendelft, MD, PhD, Atlanta, GA; Am J Clin Pathol. 2001;115:448-459.
and Members: Kenneth A. Ault, MD, South Portland, ME; Bruce
Davis, MD, South Portland, ME; Keiji Fujimoto, MEng, Kobe,
Japan; Paul Harrison, PhD, London, England; Berend Houwen,
MD, PhD, Atlanta, GA; Luc van Hove, MD, PhD, Santa Clara,
CA; Jolanta Kunicka, PhD, Tarrytown, NY; Francis Lacombe,
MD, Pessac, France; Didier Lakomsky, MD, Montpelier, France;
Sam Machin, MD, London, England; and Robert Raynor, PhD,
Miami, FL.
The use of trade names is for identification only and does not
constitute endorsement by the Public Health Service of the US
Department of Health and Human Services.
References
1. Zwart A, van Assendelft OW, Bull BS, et al. Recommen-
dations for reference method for haemoglobinometry in
human blood (ICSH standard 1995) and specifications for
international haemiglobincyanide reference preparation (4th
edition). J Clin Pathol. 1996;49:271-274.
2. International Council for Standardization in Haematology
Expert Panel on Blood Cell Sizing. Recommendation for
reference method for determination by centrifugation of
packed cell volume of blood. J Clin Pathol. 1980;33:1-2.
3. International Council for Standardization in Haematology
Expert Panel on Cytometry. Reference method for the
enumeration of erythrocytes and leucocytes. Clin Lab
Haematol. 1994;16:131-138.
4. International Council for Standardization in Haematology
Expert Panel on Cytometry. The assignment of values to fresh
blood used for calibrating automated blood cell counters. Clin
Lab Haematol. 1988;10:203-212.
5. Dacie JV, Lewis SM. Practical Haematology. 8th ed. Edinburgh,
Scotland: Churchill Livingstone; 1995.
464 Am J Clin Pathol 2001;115:460-464 © American Society of Clinical Pathologists
You might also like
- Essentials of ABO -Rh Grouping and Compatibility Testing: Theoretical Aspects and Practical ApplicationFrom EverandEssentials of ABO -Rh Grouping and Compatibility Testing: Theoretical Aspects and Practical ApplicationRating: 5 out of 5 stars5/5 (1)
- Ijpo 3 (2) 351-353Document3 pagesIjpo 3 (2) 351-353anggaririnNo ratings yet
- Tampil Jurnal Dr. Hafni FixDocument28 pagesTampil Jurnal Dr. Hafni FixCenyiqanita NurqanitaNo ratings yet
- Comparison of ABX PENTRA 120 Retic, Sysmex R-2000, Flow Cytometry, and Manual CountsDocument10 pagesComparison of ABX PENTRA 120 Retic, Sysmex R-2000, Flow Cytometry, and Manual CountsdayanaNo ratings yet
- Platelet Estimation by Manual and AutomationDocument4 pagesPlatelet Estimation by Manual and AutomationKamran DawoodNo ratings yet
- Body Fluid Cell Counts by Automated MethodsDocument11 pagesBody Fluid Cell Counts by Automated MethodsntnquynhproNo ratings yet
- Complete Blood Counts and ExaminationDocument63 pagesComplete Blood Counts and Examinationzarairahad486No ratings yet
- FINAL Automated-HematologyDocument67 pagesFINAL Automated-HematologyDineshprakash GovindhrajNo ratings yet
- Sysmex XS-1000i Automated Hematology AnalyzerDocument3 pagesSysmex XS-1000i Automated Hematology AnalyzerAdi Subagio0% (1)
- Laboratory Technology: Flow Cytometry Principles and Application in HematologyDocument40 pagesLaboratory Technology: Flow Cytometry Principles and Application in HematologyYuli RohmaNo ratings yet
- Platelet Counting by The Coulter LH 750, Sysmex XE 2100Document7 pagesPlatelet Counting by The Coulter LH 750, Sysmex XE 2100blanket_thNo ratings yet
- ZP11 Xxi 63Document8 pagesZP11 Xxi 63Jai CarungayNo ratings yet
- Advances in Platelet CountingDocument8 pagesAdvances in Platelet CountingKamran DawoodNo ratings yet
- Letter To The Editor: Comparison Between Automated and Microscopic Analysis in Body Fluids CytologyDocument3 pagesLetter To The Editor: Comparison Between Automated and Microscopic Analysis in Body Fluids CytologybalkisNo ratings yet
- Plateletcrit, Mean Platelet Volume, Platelet Distribution Width: Its Expected Values and Correlation With Parallel Red Blood Cell ParametersDocument4 pagesPlateletcrit, Mean Platelet Volume, Platelet Distribution Width: Its Expected Values and Correlation With Parallel Red Blood Cell ParametersSoumik SahaNo ratings yet
- Automated Nucleated Red Blood Cell Count Using The Mindray BC - 6800 Hematology AnalyzerDocument6 pagesAutomated Nucleated Red Blood Cell Count Using The Mindray BC - 6800 Hematology AnalyzerAngelNo ratings yet
- Alta Mimi 2012Document8 pagesAlta Mimi 2012Erika SantiagoNo ratings yet
- JR 2 AnasDocument7 pagesJR 2 AnasCitra DewiNo ratings yet
- Hema LabDocument13 pagesHema LabRothessa CaringalNo ratings yet
- Haematology Test Name Results Biological Reference Interval Units Specimen Test Method CBC - Complete Blood CountDocument8 pagesHaematology Test Name Results Biological Reference Interval Units Specimen Test Method CBC - Complete Blood CountArun DheekshahNo ratings yet
- Dacosta 2015Document36 pagesDacosta 2015JE SimianNo ratings yet
- Sysmex ScattergramsDocument7 pagesSysmex ScattergramsRuxandra Mesaros100% (1)
- Automated Blood Cell AnalysisDocument5 pagesAutomated Blood Cell AnalysiswerfsdsfNo ratings yet
- HematologyDocument12 pagesHematologyJeloMarielleEvangelistaNo ratings yet
- Hematocrit - StatPearls - NCBI BookshelfDocument4 pagesHematocrit - StatPearls - NCBI BookshelfVincent ReyesNo ratings yet
- Electrical Impedance: Hematology AnalyzerDocument2 pagesElectrical Impedance: Hematology AnalyzerAzura JKNo ratings yet
- DR Preeti Mansukhani - CBC 5 Parts - 2017Document56 pagesDR Preeti Mansukhani - CBC 5 Parts - 2017Silence T-jmNo ratings yet
- High Fluorescent Platelet FractionDocument18 pagesHigh Fluorescent Platelet Fractionswaraj sharmaNo ratings yet
- Pandey 2020Document5 pagesPandey 2020my accountNo ratings yet
- Int J Lab Hematology - 2022 - Marinov - Validation of A Single Tube 3 Colour Immature Red Blood Cell Screening Assay ForDocument7 pagesInt J Lab Hematology - 2022 - Marinov - Validation of A Single Tube 3 Colour Immature Red Blood Cell Screening Assay ForMaria SousaNo ratings yet
- Evaluation of The Ruminant Complete Blood Dell CountDocument26 pagesEvaluation of The Ruminant Complete Blood Dell CountDirección Científica Laboratorio VitalabNo ratings yet
- Platelet Distribution Curves PDFDocument6 pagesPlatelet Distribution Curves PDFsumathiNo ratings yet
- Automation in HaematologyDocument24 pagesAutomation in HaematologyGianna SablanNo ratings yet
- Diagnostics 12 00068Document9 pagesDiagnostics 12 00068Nyuori CosmasNo ratings yet
- Clinical Case Report - XS-1000i - SpanishDocument45 pagesClinical Case Report - XS-1000i - SpanishLaboratorios Clinicos ZaragozaNo ratings yet
- Hema Activity 3Document5 pagesHema Activity 3Nico LokoNo ratings yet
- Int J Lab Hematology - 2007 - ZANDECKI - Spurious Counts and Spurious Results On Haematology Analysers A Review Part IDocument17 pagesInt J Lab Hematology - 2007 - ZANDECKI - Spurious Counts and Spurious Results On Haematology Analysers A Review Part IYangnuu TitusNo ratings yet
- Automated Cell Counting InstrumentationDocument33 pagesAutomated Cell Counting InstrumentationCecille AnnNo ratings yet
- Xtra Online Platelet Distribution CurvesDocument6 pagesXtra Online Platelet Distribution CurvesEslam MaherNo ratings yet
- Five-Part Hematology AnalyzerDocument78 pagesFive-Part Hematology Analyzerswaraj sharmaNo ratings yet
- File - Report (1665892839610)Document2 pagesFile - Report (1665892839610)Sameer KauraNo ratings yet
- 173 FullDocument13 pages173 FullYaser MNo ratings yet
- Hematology Analyzer (Coulter Method)Document22 pagesHematology Analyzer (Coulter Method)Sanchia Janita Khodijah100% (1)
- Basic HematologyDocument89 pagesBasic Hematologydrafq2000No ratings yet
- Introduction To HematologyDocument11 pagesIntroduction To HematologyJustin Louie EstradaNo ratings yet
- Erroneous Automated Optical Platelet Counts PDFDocument8 pagesErroneous Automated Optical Platelet Counts PDFfar faraNo ratings yet
- Flow cytometry and red cell indices analysisDocument4 pagesFlow cytometry and red cell indices analysisKINGSCOMPUTERS CYBERNo ratings yet
- 4 - Hematology UnitDocument16 pages4 - Hematology UnitMary CabalceNo ratings yet
- Hemapheresis: Sandhya R. Panch and Harvey G. KleinDocument11 pagesHemapheresis: Sandhya R. Panch and Harvey G. KleinNur Melani Sari WardaniNo ratings yet
- PLT MeasurementsDocument6 pagesPLT MeasurementsAudreySlitNo ratings yet
- New QuantitiesDocument6 pagesNew Quantities20100117 Bùi Thùy VyNo ratings yet
- Dimal HemaDocument8 pagesDimal HemaGioAndrew ReyesNo ratings yet
- Science: Assessment of The Reliability of The Sysmex XE-5000 Analyzer To Detect Platelet ClumpsDocument6 pagesScience: Assessment of The Reliability of The Sysmex XE-5000 Analyzer To Detect Platelet ClumpsnivmastNo ratings yet
- Group 6 Hema2 Exp 2Document3 pagesGroup 6 Hema2 Exp 2Aaron Tzergio GotohNo ratings yet
- Evaluation of The Performance of Sysmex XN-3100 Automated Hematology Analyzer Regarding The Sysmex XE-2100 and Microscopic ExaminationDocument9 pagesEvaluation of The Performance of Sysmex XN-3100 Automated Hematology Analyzer Regarding The Sysmex XE-2100 and Microscopic ExaminationbalkisNo ratings yet
- Peripheral Smear Review: Bluegrass Community Hospital Laboratory 360 Amsden Avenue Versailles, Kentucky 40383Document4 pagesPeripheral Smear Review: Bluegrass Community Hospital Laboratory 360 Amsden Avenue Versailles, Kentucky 40383Gaby GómezNo ratings yet
- Lab Report 2157646 20210113110650Document3 pagesLab Report 2157646 20210113110650marlon molinaNo ratings yet
- Int J Lab Hematology - 2020 - Buttarello - Reticulated Platelets and Immature Platelet Fraction Clinical Applications andDocument8 pagesInt J Lab Hematology - 2020 - Buttarello - Reticulated Platelets and Immature Platelet Fraction Clinical Applications andMarcellia AngelinaNo ratings yet
- Evaluation of The Abbott CELL-DYN 4000 HematologyDocument10 pagesEvaluation of The Abbott CELL-DYN 4000 Hematologypasamuco473No ratings yet
- Hema I Chapter 10 - PCVDocument24 pagesHema I Chapter 10 - PCVchris andrieNo ratings yet
- AIPMT BIOLOGY Study Material PDFDocument346 pagesAIPMT BIOLOGY Study Material PDFDanish BoddaNo ratings yet
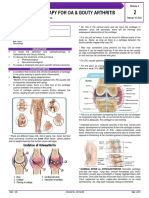
- Pharma - M6L2 - Drug Therapy For OA and Gouty ArthritisDocument8 pagesPharma - M6L2 - Drug Therapy For OA and Gouty ArthritisAngelaTrinidadNo ratings yet
- Arctium LappaDocument2 pagesArctium LappaIkkasama NaguenneNo ratings yet
- MP State Level Combined Counselling-2016 for MBBS/BDS Allotment List (First RoundDocument644 pagesMP State Level Combined Counselling-2016 for MBBS/BDS Allotment List (First RoundAnmol ShivamNo ratings yet
- Hormone Treatment Boosts Growth of Siam Catfish LarvaeDocument9 pagesHormone Treatment Boosts Growth of Siam Catfish LarvaeNissa SissariNo ratings yet
- JAIPUR Artificial LIMBS-Social EntrepreneurshipDocument8 pagesJAIPUR Artificial LIMBS-Social Entrepreneurshipeapen123No ratings yet
- History of OrthodonticsDocument133 pagesHistory of OrthodonticsAnubha VermaNo ratings yet
- Medical Diagnosis PDFDocument30 pagesMedical Diagnosis PDF99nonameNo ratings yet
- Poisons of The Realm: RPG Netbook.Document38 pagesPoisons of The Realm: RPG Netbook.Canageek100% (1)
- A Simple and Reliable Submental Intubation.68Document4 pagesA Simple and Reliable Submental Intubation.68Tîrban Pantelimon FlorinNo ratings yet
- PMAD PresentationDocument17 pagesPMAD PresentationBrittany100% (1)
- Msds Titipan1Document5 pagesMsds Titipan1anitacahyaNo ratings yet
- Herkin-3000 Skin Care System ManualDocument19 pagesHerkin-3000 Skin Care System ManualEnigma International IncNo ratings yet
- Contraception 2017Document85 pagesContraception 2017pt.mahmoudNo ratings yet
- Chapter 7Document34 pagesChapter 7Hima AlqahtaniNo ratings yet
- Breath of FireDocument3 pagesBreath of FirepraveenmspkNo ratings yet
- Emergency DrugsDocument19 pagesEmergency DrugsAdy PutroNo ratings yet
- Data Obat Terjual Jan-Des 2018Document62 pagesData Obat Terjual Jan-Des 2018HanNo ratings yet
- Pediatric SMLE NotesDocument25 pagesPediatric SMLE NotesMarwa Tariq Ahmed Abdulla Ahmed Al MurbatiNo ratings yet
- Exposure Chart Mobile X Ray PDFDocument8 pagesExposure Chart Mobile X Ray PDFlutfiaNo ratings yet
- Endocrine HypertensionDocument43 pagesEndocrine HypertensionAlessia GuerraNo ratings yet
- Klasifikasi Derajat Keparahan Gigitan UlarDocument2 pagesKlasifikasi Derajat Keparahan Gigitan UlarFransiska SariNo ratings yet
- Psychiatry: Mental State ExaminationDocument3 pagesPsychiatry: Mental State ExaminationSok-Moi Chok100% (3)
- Branches of PsychologyDocument2 pagesBranches of Psychologyanandi rayNo ratings yet
- Types and Treatment of LeukemiaDocument14 pagesTypes and Treatment of LeukemiaalirezaNo ratings yet
- Annex To The European Commission Guideline On Excipients in The Labelling and Package Leaflet of Medicinal Products For Human Use' (2019)Document21 pagesAnnex To The European Commission Guideline On Excipients in The Labelling and Package Leaflet of Medicinal Products For Human Use' (2019)janoscribdNo ratings yet
- Significance of HACCP and SSOP in Food Processing EstablishmentsDocument7 pagesSignificance of HACCP and SSOP in Food Processing EstablishmentselfiraNo ratings yet
- 2009 Annual ReportDocument12 pages2009 Annual ReportWSCFoundationNo ratings yet
- Symposium Topic: Current Update in Diagnosis & Management of Emergency MedicineDocument2 pagesSymposium Topic: Current Update in Diagnosis & Management of Emergency MedicineAfriza AnggriniNo ratings yet
- Ultrasonographic Diagnosis of Pregnancy in Dogs and CatsDocument8 pagesUltrasonographic Diagnosis of Pregnancy in Dogs and CatsAnur SinglaNo ratings yet
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionFrom EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionRating: 4 out of 5 stars4/5 (402)
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (14)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 5 out of 5 stars5/5 (78)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 3.5 out of 5 stars3.5/5 (2)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsFrom EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsRating: 5 out of 5 stars5/5 (1)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsFrom EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNo ratings yet
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsFrom EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsRating: 4.5 out of 5 stars4.5/5 (169)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeFrom EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeNo ratings yet
- Techniques Exercises And Tricks For Memory ImprovementFrom EverandTechniques Exercises And Tricks For Memory ImprovementRating: 4.5 out of 5 stars4.5/5 (40)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisFrom EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisRating: 4 out of 5 stars4/5 (1)
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 5 out of 5 stars5/5 (4)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsFrom EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsRating: 3.5 out of 5 stars3.5/5 (3)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisFrom EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisRating: 4.5 out of 5 stars4.5/5 (42)
- The Ultimate Guide To Memory Improvement TechniquesFrom EverandThe Ultimate Guide To Memory Improvement TechniquesRating: 5 out of 5 stars5/5 (34)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 5 out of 5 stars5/5 (5)
- The Happiness Trap: How to Stop Struggling and Start LivingFrom EverandThe Happiness Trap: How to Stop Struggling and Start LivingRating: 4 out of 5 stars4/5 (1)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.From EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Rating: 4.5 out of 5 stars4.5/5 (110)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsFrom EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsNo ratings yet
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessFrom EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessRating: 4.5 out of 5 stars4.5/5 (327)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeFrom EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeRating: 4.5 out of 5 stars4.5/5 (253)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 3.5 out of 5 stars3.5/5 (33)
- Daniel Kahneman's "Thinking Fast and Slow": A Macat AnalysisFrom EverandDaniel Kahneman's "Thinking Fast and Slow": A Macat AnalysisRating: 3.5 out of 5 stars3.5/5 (130)
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisFrom EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisRating: 5 out of 5 stars5/5 (3)