Professional Documents
Culture Documents
CARABAPENEMS
CARABAPENEMS
Uploaded by
Shuaib Manisur0 ratings0% found this document useful (0 votes)
14 views14 pagesThe document discusses early and newer carbapenems. It describes the structures and characteristics of thienamycin, imipenem-cilastatin, meropenem, and biapenem. Key points covered include the structural features shared by carbapenems and other beta-lactam antibiotics, and how modifications at positions 1 and 2 of the carbapenem nucleus provide stability and affect antibacterial spectrum.
Original Description:
Pharmchem
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses early and newer carbapenems. It describes the structures and characteristics of thienamycin, imipenem-cilastatin, meropenem, and biapenem. Key points covered include the structural features shared by carbapenems and other beta-lactam antibiotics, and how modifications at positions 1 and 2 of the carbapenem nucleus provide stability and affect antibacterial spectrum.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
14 views14 pagesCARABAPENEMS
CARABAPENEMS
Uploaded by
Shuaib ManisurThe document discusses early and newer carbapenems. It describes the structures and characteristics of thienamycin, imipenem-cilastatin, meropenem, and biapenem. Key points covered include the structural features shared by carbapenems and other beta-lactam antibiotics, and how modifications at positions 1 and 2 of the carbapenem nucleus provide stability and affect antibacterial spectrum.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 14
GROUP THREE
• MATENDE HUSSEIN DPH/8548/171/DU
• NAKKAZI KIZZA JOAN DPH/8862/172/DU
• NANTONGO DOREEN DPH/8998/172/DU
• TWINOMUHANGI CALEB DPH/6630/163/DU
• HOPE CECILIA DPH/9083/172/DU
• NAGUJJA BABIRYE PATIENCE DPH/9082/172/
DU
• KAMUHANDA DANIEL DPH/8917/172/DU
• WANJALA PETER DPH/6130/162/DU
• KIKOMBERWA GEOFREY DPH/7077/163/DU
• JASSA FREDRICK DPH/6936/163/DU
CARBAPENEMS
CLASSIFICATION
EARLY CARBAPENEMS
1. THIENAMYCIN
• Thienamycin is a novel -lactam antibiotic
first isolated from fermentation of cultures
of Streptomyces cattleya.
STRUCTURE OF THIENAMYCIN
• Two structural features of thienamycin
are shared with the penicillins and
cephalosporins:
• A fused bicyclic ring system containing a
beta-lactam.
• An equivalently attached 3-carboxyl group.
• The bicyclic system consists of a
carbapenem containing a double bond
between C-2 and C-3
Structure of thienamycin
SAR of Thienamycin
The double bond in the bicyclic structure creates
considerable ring strain and increases the reactivity of
the beta-lactam to ring opening reactions.
A simple 1-hydroxyethyl group is essential(provides
resistance to lactamases).
A 2-aminoethylthioether function at C-2.
At its optimally stable pH between 6 and 7
thienamycin undergoes concentration-dependent
inactivation. This inactivation is believed to result from
intermolecular aminolysis of the beta lactam by the
cysteamine side chain of a second molecules.
NB: Susceptibility to hydrolytic inactivation by renal
dehydropeptidase-1(DHP-1) which causes it to have an
unacceptably short half-life in vivo.
2. IMIPENEM-CILASTATIN.
Imipenem is N-formimidoylthienamycin, the
most successful of a series of chemically stable
derivatives of thienamycin in which the primary
amino group is converted to a nonnucleophilic
basic function.
Cilastatin is an inhibitor of DHP-1
Imipenem is very stable to most beta-
lactamases.
It is an inhibitor of beta-lactamases from certain
Gram-negative bacteria resistant to other -beta
lactam antibiotics e.g. P. aeruginosa,
IMIPENEM-CILASTATIN
• structure
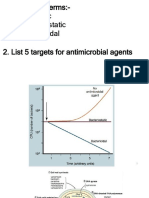
NEWER CARBAPENEMS:
• In the design of new carbapenems, structural
variations are being investigated in the objective of
developing analogs in advantage over imipenem.
• Improvements that are particularly desired include;
• Stability to hydrolysis catalysed by DHP-1.
• Stability to bacterial metallo-beta
lactamases(carbapenems) that hydrolyse imipenem.
• Activity against MRSA and increased potency
against P. aeruginosa especially imipenem resistant
strains.
• Enhanced PK properties such as oral bioavailability
and long duration of action, have therefore received
little emphasis in carbapenem analog design.
NEWER CARABAPENEMS
a) MEROPENEM
Meropenem is a second-generation
carbapenem that, to date, has undergone the
most extensive clinical evaluation.
Meropenem exhibits greater potency against
Gram-negative and anaerobic bacteria than
does imipenem, but it is slightly less active
against most Gram-positive species.
Meropenem is not hydrolyzed by DHP-I and is
resistant to most B-lactamases.
STRUCTURE OF MEROPENEM
b) BIAPENEM
• Biapenem is a newer second-generation
carbapenem with chemical and
microbiological properties similar to those
of meropenem.
• Biapenem is stable to DHP-I and resistant
to most B-lactamases.
• It is not active orally.
Structure of biapenem
SAR OF NEWER CARBAPENEM:
• Modifications, therefore have concentrated
on variations at positions 1 and 2 of the
carbapenem nucleus.
• The incoporation of a B-methyl group at the
position one gives the carbapenem stability
to hydrolysis by renal DHP-1.
• Substituents at the 2-position affects
primarily the spectrum of antibacterial
activity of carbapenem influencing
penetration into bacteria.
SAR Contd
• The capability of carbapenems to exist as
zwitterionic structures ,resulting from the
combined features of a basic amine
function attached to the 2-position and 3-
carboxyl group, may enable these
carbapenems to enter bacteria via their
charged porin chanels.
You might also like
- Summary For Antibiotic For USMLE Exam - USMLE MATERIALS - Updated USMLE Study DataDocument5 pagesSummary For Antibiotic For USMLE Exam - USMLE MATERIALS - Updated USMLE Study Dataomy yadavNo ratings yet
- Classification of AntibioticsDocument5 pagesClassification of AntibioticsdenaNo ratings yet
- Antibiotic Resistance: Dr. Sharifah Shakinah Supervisor: Dr. Eppy SPPD KptiDocument25 pagesAntibiotic Resistance: Dr. Sharifah Shakinah Supervisor: Dr. Eppy SPPD KptiShasha Shakinah100% (1)
- AntibioticsDocument53 pagesAntibioticsMaheen IdreesNo ratings yet
- Beta Lactam AntibioticsDocument28 pagesBeta Lactam AntibioticsHassan.shehri100% (11)
- Cell Wall Inhibitor PPT SlideDocument47 pagesCell Wall Inhibitor PPT Slidekhawaja sahabNo ratings yet
- Beta Lactams LectureDocument59 pagesBeta Lactams Lecturexmd6787qpqNo ratings yet
- Nelson's Pediatric Antimicrobial Therapy 24th Edition 2018Document325 pagesNelson's Pediatric Antimicrobial Therapy 24th Edition 2018Eduardo Rios Dubois100% (2)
- EsblDocument16 pagesEsblsharmamaddy32No ratings yet
- Antibiotics Lecture 3 - CarbapenemsDocument22 pagesAntibiotics Lecture 3 - CarbapenemsRenas SalayNo ratings yet
- Unit 1 - MC 3Document58 pagesUnit 1 - MC 3Shreyas ShreyuNo ratings yet
- Articulo 3Document5 pagesArticulo 3Karen CaizaNo ratings yet
- Week 4 - Penicillins, CephalosporinsDocument44 pagesWeek 4 - Penicillins, CephalosporinsTiko JomidavaNo ratings yet
- Meropenem Laboratory and Clinical DataDocument9 pagesMeropenem Laboratory and Clinical DataADINo ratings yet
- 2.2.5 - Cell Wall Inhibitors - Carbapenems - 090ct2012-Oct 2014Document9 pages2.2.5 - Cell Wall Inhibitors - Carbapenems - 090ct2012-Oct 2014tresorstephane669No ratings yet
- Mechanism of Drug ResistanceDocument105 pagesMechanism of Drug ResistanceHimani AggarwalNo ratings yet
- Lecture 7 Chemotherapeutic AgentDocument54 pagesLecture 7 Chemotherapeutic AgentjowditzNo ratings yet
- Laboratory of Microbiology Medical Faculty UB 2009Document37 pagesLaboratory of Microbiology Medical Faculty UB 2009zianaNo ratings yet
- Mark Miguel P. Latras, RPHDocument11 pagesMark Miguel P. Latras, RPHLOLOLONo ratings yet
- Antibiotics That Are Used As ProdrugDocument48 pagesAntibiotics That Are Used As ProdrugApurba Sarker Apu100% (1)
- Antibacterials II - HP 2019 - StudentDocument83 pagesAntibacterials II - HP 2019 - StudentKayleigh MastersNo ratings yet
- Hetrocyclic Rings of Carbapenems AntibioticsDocument5 pagesHetrocyclic Rings of Carbapenems Antibioticsعبدالرحمن بسمان غانمNo ratings yet
- Week 5 - Beta Lactams and OthersDocument38 pagesWeek 5 - Beta Lactams and OthersTiko JomidavaNo ratings yet
- Antimicrobialchemotheray PDFDocument80 pagesAntimicrobialchemotheray PDFغمدان دماج الحمزيNo ratings yet
- 20-08-21 - T.Y - Ict - 21-22 - Chap 2 - 3 - Antibiotics and Principles of Antibacterial Agents - SulfonamidesDocument24 pages20-08-21 - T.Y - Ict - 21-22 - Chap 2 - 3 - Antibiotics and Principles of Antibacterial Agents - SulfonamidesRahul LakhaniNo ratings yet
- Antibiotics PDFDocument49 pagesAntibiotics PDFgautamtajesh1983No ratings yet
- Antimicrobial Chemotherapy IDocument30 pagesAntimicrobial Chemotherapy Inighat khanNo ratings yet
- 21oct Copy 150118071331 Conversion Gate02 PDFDocument80 pages21oct Copy 150118071331 Conversion Gate02 PDFKarina NilasariNo ratings yet
- AntibioticsDocument115 pagesAntibioticspmm21d229No ratings yet
- Beta Lactams 1Document44 pagesBeta Lactams 1C E princeyNo ratings yet
- Antimicrobial Drugs: Laboratory of Microbiology Medical Faculty Brawijaya UniversityDocument38 pagesAntimicrobial Drugs: Laboratory of Microbiology Medical Faculty Brawijaya UniversityYuu Ayu'k LifestarNo ratings yet
- AntibioticsDocument32 pagesAntibioticsParamanand SinghNo ratings yet
- Cell Wall InhibitorDocument99 pagesCell Wall InhibitorAdeniran CharlesNo ratings yet
- Beta Lactam AntibioticsDocument23 pagesBeta Lactam Antibioticsali al eisaNo ratings yet
- Rho Chi Tutoring Session: Med Chem Exam I: February 1st, 2021 Carly Huggins and Katey JamesDocument43 pagesRho Chi Tutoring Session: Med Chem Exam I: February 1st, 2021 Carly Huggins and Katey JamesMorrigan DearmanNo ratings yet
- Lecture 5 6 Antibacterials Protien & NA Synthesis InhibitorsDocument23 pagesLecture 5 6 Antibacterials Protien & NA Synthesis InhibitorsyomifNo ratings yet
- Pharmacology of Cephalosporins: General Overview: Flavio Guzmán, M.DDocument21 pagesPharmacology of Cephalosporins: General Overview: Flavio Guzmán, M.DerikandzaksmomNo ratings yet
- Antibacterial CombinationsDocument21 pagesAntibacterial CombinationsshaitabliganNo ratings yet
- Antibacterial: Topics: 1. Cell Wall Synthesis InhibitorDocument26 pagesAntibacterial: Topics: 1. Cell Wall Synthesis InhibitorMusfira YaseenNo ratings yet
- Medicinal Chemistry IV Antibiotics: Cephalosporins: Sam DawbaaDocument7 pagesMedicinal Chemistry IV Antibiotics: Cephalosporins: Sam DawbaaAhmed BajahNo ratings yet
- Tabel MeroDocument12 pagesTabel MeroNurina yupiNo ratings yet
- Mechanisms of Antibiotic Resistance: DR T. Aswani Ndonga MSC Tid I April 2010Document25 pagesMechanisms of Antibiotic Resistance: DR T. Aswani Ndonga MSC Tid I April 2010GAMING MONKEYNo ratings yet
- PenicillinsDocument15 pagesPenicillinsFaisalNo ratings yet
- MonobactamDocument18 pagesMonobactamVALENTINA ZAMBRANO ROMERONo ratings yet
- Recent Developments in Carbapenems: ReviewDocument16 pagesRecent Developments in Carbapenems: ReviewFrancielleNo ratings yet
- B-Lactam AntibioticsDocument16 pagesB-Lactam AntibioticsDrx Harish PatelNo ratings yet
- Antimalarial DrugsDocument36 pagesAntimalarial DrugsKasim UmarNo ratings yet
- Beta-Lactam Antibiotics Kul Nov 2014Document44 pagesBeta-Lactam Antibiotics Kul Nov 2014Sahala Louis AlexanderNo ratings yet
- Beta-Lactam Antibiotics Will Bind To Serine Proteases (TranspeptidaseDocument4 pagesBeta-Lactam Antibiotics Will Bind To Serine Proteases (TranspeptidaseJames RussellNo ratings yet
- Biseptol & IsoniazidDocument61 pagesBiseptol & IsoniazidYeshaa MiraniNo ratings yet
- Antibacterial AntibioticsDocument13 pagesAntibacterial AntibioticsMuhamed ArsalanNo ratings yet
- Resistance To AntimicrobialsDocument5 pagesResistance To AntimicrobialsLuqman Al-Bashir FauziNo ratings yet
- Medicinal Chemistry of Beta-Lactam AntibioticsDocument13 pagesMedicinal Chemistry of Beta-Lactam AntibioticsJosiah O OmobaNo ratings yet
- Antibiotics: Means Against LifeDocument13 pagesAntibiotics: Means Against Lifeshankul kumar100% (1)
- Antimicrobcell Wall InhibitorsDocument21 pagesAntimicrobcell Wall Inhibitorsymeen9829No ratings yet
- Chemotherapy NotesDocument9 pagesChemotherapy Notesnileshkumarhjoshi942No ratings yet
- Anti T.B DrugsDocument120 pagesAnti T.B DrugsromalaramNo ratings yet
- Antituberculosis DrugsDocument31 pagesAntituberculosis DrugsF ParikhNo ratings yet
- Aminoglycosides (17.07.2017)Document44 pagesAminoglycosides (17.07.2017)Habibul Kowser (Rishat)No ratings yet
- Principles of Antimicrobial TherapyDocument180 pagesPrinciples of Antimicrobial TherapyJannnat JabbbarNo ratings yet
- 7610 (24) Antimicrobial Drugs I (Antibiotics) - UpdatedDocument59 pages7610 (24) Antimicrobial Drugs I (Antibiotics) - UpdatedAli AlhayaliNo ratings yet
- Antimycobacterial Agents: Dr. Amal BelaidDocument40 pagesAntimycobacterial Agents: Dr. Amal BelaidMustafa RihanNo ratings yet
- Beta-Lactam Antibiotics & Other Inhibitors of Cell WallDocument71 pagesBeta-Lactam Antibiotics & Other Inhibitors of Cell WallAlvin LaurenceNo ratings yet
- Antibiotics Targeting Cell Wall Synthesis in BacteriaDocument4 pagesAntibiotics Targeting Cell Wall Synthesis in BacteriaMuhammad NawazNo ratings yet
- Amp CDocument24 pagesAmp CRajkishor YadavNo ratings yet
- Daftar Atc DDD Antibiotik Who 2018Document12 pagesDaftar Atc DDD Antibiotik Who 2018APOTEKER RSUMMNo ratings yet
- Antimikroba Anti Jamur Antiparasit Antibiotik Antiviral Antimico-Bacterium Antimikosis Antihelmintik Antiamuba AntimalariaDocument22 pagesAntimikroba Anti Jamur Antiparasit Antibiotik Antiviral Antimico-Bacterium Antimikosis Antihelmintik Antiamuba AntimalariaHaris GaulNo ratings yet
- Cell Wall Synthesis - BacterialProteinSynthesis - AntimicrobialsDocument99 pagesCell Wall Synthesis - BacterialProteinSynthesis - AntimicrobialsJoslin Roz GalileaNo ratings yet
- EUCAST Sabloane 2022Document11 pagesEUCAST Sabloane 2022MirelaNo ratings yet
- AntibioticsDocument102 pagesAntibioticsotto vansandersNo ratings yet
- Penicillins - KatzungDocument6 pagesPenicillins - KatzungKarl CNo ratings yet
- UntitledDocument24 pagesUntitledRicardo silvestreNo ratings yet
- Antibiotic Class by CoverageDocument3 pagesAntibiotic Class by Coverageayy1No ratings yet
- Antimicrobial Table of Bacteria: CLSI 2017Document16 pagesAntimicrobial Table of Bacteria: CLSI 2017behzad bm0% (1)
- Pharmacology Assignment 2Document3 pagesPharmacology Assignment 2Shamantha Santhana KrishnanNo ratings yet
- Morganella SPP (Morganella Morganii)Document15 pagesMorganella SPP (Morganella Morganii)Dian Jean Cosare GanoyNo ratings yet
- Saldo Penerimaan & Pengeluaran07 - Maret - 2021Document34 pagesSaldo Penerimaan & Pengeluaran07 - Maret - 2021Desi Yuliana HarahapNo ratings yet
- Antibiotic Reference GuideDocument2 pagesAntibiotic Reference GuideDreyden HaloNo ratings yet
- Master PSL Kevin Car I Ou 2021Document54 pagesMaster PSL Kevin Car I Ou 2021emma melottiNo ratings yet
- The Place of New Antibiotics For Gram-Negative Bacterial Infections in Intensive Care - Report of A Consensus ConferenceDocument12 pagesThe Place of New Antibiotics For Gram-Negative Bacterial Infections in Intensive Care - Report of A Consensus ConferenceStacey WoodsNo ratings yet
- 1 3 19Document165 pages1 3 19Nurhayati AmatillahNo ratings yet
- Daftar DDDDocument6 pagesDaftar DDDelisemilNo ratings yet
- Chapter 43 - Beta-Lactam Antibiotics & Amp Other Cell Wall - & Amp Membrane-Active AntibioticsDocument9 pagesChapter 43 - Beta-Lactam Antibiotics & Amp Other Cell Wall - & Amp Membrane-Active AntibioticsSara OchoaNo ratings yet
- ASCIA HP Penicillin Allergy Guide 2016Document1 pageASCIA HP Penicillin Allergy Guide 2016kkkssbbNo ratings yet
- Antibotics Venn DiagramDocument2 pagesAntibotics Venn DiagramTop VidsNo ratings yet
- Lap or AnDocument420 pagesLap or Annelle73No ratings yet
- Carbapenem - Sparing - Strategy - Carbapenemase, Treatment and ASP. COID 2019Document11 pagesCarbapenem - Sparing - Strategy - Carbapenemase, Treatment and ASP. COID 2019Candy RevolloNo ratings yet
- Beta Lactam AntibioticsDocument15 pagesBeta Lactam AntibioticsNiharika ModiNo ratings yet
- Esbl GNBDocument4 pagesEsbl GNBPutri Nilam SariNo ratings yet