100% found this document useful (1 vote)
641 views6 pages1.mechanism of Polymerization
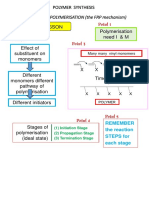
The document discusses different types of polymers and their classification. It describes natural and synthetic polymers, thermoplastics and thermosetting polymers, addition and condensation polymers, elastomers, fibers, resins and plastics, and homopolymers and copolymers. It also summarizes the general methods of polymer production, including addition polymerization, condensation polymerization, and different mechanisms of polymerization such as free radical, cationic, anionic, and coordination polymerization.
Uploaded by
Shalynee SuthaharCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
100% found this document useful (1 vote)
641 views6 pages1.mechanism of Polymerization
The document discusses different types of polymers and their classification. It describes natural and synthetic polymers, thermoplastics and thermosetting polymers, addition and condensation polymers, elastomers, fibers, resins and plastics, and homopolymers and copolymers. It also summarizes the general methods of polymer production, including addition polymerization, condensation polymerization, and different mechanisms of polymerization such as free radical, cationic, anionic, and coordination polymerization.
Uploaded by
Shalynee SuthaharCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd