Professional Documents
Culture Documents
Measurement of Diffusivity of Organic Liquids Through Polymer Membranes
Uploaded by
LorenaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Measurement of Diffusivity of Organic Liquids Through Polymer Membranes
Uploaded by
LorenaCopyright:
Available Formats
Measurement of Diffusivity of Organic Liquids through
Polymer Membranes
A Simple and Inexpensive Laboratory Experiment
U. Shanthamurthy Aiial and Tejraj M. Aminabhavi'
Karnatak University. Dharwad, India 580 003
A common laboratory experiment to study the interaction ation processes in a polymer above its glass transition tem-
of polymer membranes with organic liquids is of great rele- perature (T,) are mainly governed by the segmental mobility
vance in chemical industries and allied areas in view of the of the chains, which in turn are considered to be affected by
fact that the real tests of measuring the rate of absorption of the total free volume and its distribution within the polymer
a liquid by large pieces of polymer samples are highly time- matrix (2). In rubbery polymers well above their T,, the
consuming and often expensive. Thus, characterization of polymer chains adjust so rapidly to the presence of pene-
liquid interaction with polymer membranes by using simple trant that they do not cause diffusion anomalies. For such
laboratory tests using a small sample of polymer membrane situations the diffusion process is generally described by
is certainlv
-~~~ ~
-
" of ereat value in understanding the actual behav-
ior of a large industrial rubber sample.
Fick's theorv and is often controlled by a concentration-
independent diffusivity. One of the easiest and simplest
Theoreticallv. the contact of polvner membranes with ~rocedurestoestablish the transport mode within apolymer
organic liquidscan be described by absorption and diffusion membrane is to analyze the sorption data and thereby esti-
ohenomena ( 1 ) . It is essential to know at what rate the liquid mate the numerical value of the exponent n of eq 1.
biffuses inti the polymer matrix. Often simple laboratory MJM. = k t " (1)
methods such as sorption experiments yield useful informa-
tion on the transport characteristics of polymer membranes. Here, M,is the mass uptake at time t, M , is the equilibrium
Here we describe a simple and inexpensive laboratory ex- value, and k is a constant that depends on the structural
periment to study the diffusion of organic liquids through characteristics of the polymer in addition to its interaction
polymer membranes. This experiment can be performed by with the solvent. The value of n determines the type of
most undergraduate physical chemistry students. This pro- transport mechanism (3.4). A value of n = 0.5 suggests the
cedure helps to assess the resistivity of a polymer toward Fickian mode, whereas for the uon-Fickian diffusion n is
well-known, commonly available organic liquids like ben- --
unitv. Often. n mav v a n between 0.5 to 1. and this suggests
zene, toluene, mesitylene, cblorobenzene, n-hexane, and the Lomalo& diffusion pattern.
carbon tetrachloride. Interactions of these liquids with an The solvent transport in a polymer membrane has been
industrial polymer membrane, namely polyurethane, are be- described (2) bv Fick's second law of diffusion. which for a
ing investigated. constant diffnsrvity D is given as
Theory
Liquid absorption by polymer membranes is considered to
be a diffusion process. The diffusion of liquids and relax- where c is the concentration of the diffusing material (gI100
gof original sample) in the direction of x coordinate axis and
t is diffusion time (s). Equation 2 upon integration gives the
' Author to whom correspondence should be addressed. percentage fractional mass uptake Q(t) as
82 Journal of Chemical Education
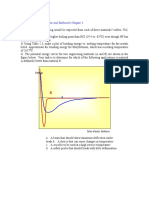
Treatment of Data
The sorption data are interpreted in terms of both percent
uptake Q(t) and percent increase in thickness h(t) of the
where h is membrane thickness. T h e first term of the inte- membrane versus square root of time, t112.~ h e s plots,
e as are
grated infinite series can be used to determine the maximum given in Figures 1and 2, exhibit linearity during early stages
absorption; D of the penetrant within the polymer matrix for of absorption [up to -50% of either Q(t) or h(t)]. From the
values of Q(t) u p to 50% can he calculated from the slope ( 8 ) slopes of the linear portions of the curves of Q(t) or h(t)
of the straight line of the plot of Q(t) versus t1I2and hy using versus tlR, diffusivities are calculated using eqs 4 and 6,
respectively, and these data, when compared (see Table l ) ,
I I I I
0 Benzene Chlorobenzene
Often attempts have been made to calculate the theoretical
sorption curves from eq 3 using D as obtained from eq 4. o Toluene 8 Carbon tetrachloridt
Another useful approach (5-7) for studying diffusivity is A Mesitylene A n-Hexane
through the dimensional response of the membrane: this
approach is based on the of "hygroelasti&ty",
where one is confronted with questions such as how much
the polymer swells and what is the magnitude of the internal
stress that develops in it. Thus, hygroelasticity can be treat-
ed by examining the relative dimensional change in relation
t o absorption, which may be expressed in a number of ways.
Expressing liquid absorption in terms of the relative weight
gain yields the definition of "coefficient of hygroe1asticity"p
*=- Ahlh,
AWIW,
where Ahlh, and AWIW, are. res~ectivelv. the relative
change in thkkness and weight' of &e polymer membrane.
The coefficient fi can be determined from the slope of the
curve relating thickness change to weight change. For many
polymer-solvent systems, p is independent of AWIW, and
may he regarded a s a constant throughout the swelling pro-
cess. Such considerations lead to the following relation for
diffusivity:
Figure 1. Variations of percent maos uptake 40 of solvem wRh square rwt of
time (t'I2). Symbols: 0, benzene; 0, toluene: A, mesitylene; 0 , chlwoben-
Zene; B, w b o n tetrachiaide; A, rrhexane,
where 9, is the slope obtained from the initial linear portions
o f t h e plot h(t) I-(Ahlh,)1001 versus 1' ".and h, is themaxi-
mum thickness: 0 Benzene Chlorobenzene
Several experiments on a wide variety of polymer-solvent Carbon tetrachloride
35
systems (8) tell us that eqs 4 and 6 give nearly identical
results on diffusivities through polymer membranes, and A Mesitylene A n-Hexane
this approach will be demonstrated in this paper.
Experimental Procedures
Any elastomer available as a rubhery material at room tempera-
ture may be used for experimentation. In this investigation,due to
the widespread use of polyurethane in industry and engineering (9),
we have employed a commercially availshle polyurethane. The base
polymer was a Vibrathane B600 (Uniroyal)cured with 4,4'-methyl-
ene-bis-o-chloroaniline (MOCA). A sample of uniform thickness
(0.250 cm) was used throughout; these were cut into circular pieces
of diameter 1.9 cm, weighing approximately 0.8 g. These should be
dried in vacuum oven at room temperature before experimentation.
The solvents, namely benzene, toluene, mesitylene, chloroben-
zene, n-hexane, and carbon tetrachloride, were obtained in their
highest purity and were double-distilled to ascertain extreme puri-
ty. Caution should be exercised in handling these chemicals. As
many of these are highly toxic, the experimentsshould be conduct-
ed under hood, with eztrerne core.
Sorption experiments were performed by placing the cut polymer
membranes in the respective liquids in screw-tight metal-capped
test hottles maintained at 25 OC (10.5 "C). At regular time intervals
the samples were removed from the test containers, blotted with
Kimwipes to remove the surface-adhered excess liquid. These were
then weighed (10.05 mg) under closed environments, the thiek-
nesses were measured (10.01 em) at several points by means of a Figure 2. Variatimofpercem increase in mickness Wnot ths memocans w m
micrometer and then placed hack into the containers. sqme rwt ol time 0' 4. Symbols have ths same meanmg as in Figne 1.
Volume 67 Number 1 January 1990 83
Table 1. Solvent Properties and DlWuslvRy Data
DiffusioncDefficlent
Molar Exponent D x lo' (cm2/5) Maximum Maximum
volume (4 by wt. gain by hygmelasticily wt. gain thickness
Penetrant (cm3/mol) eq 1 eq 4 eq 6 M. (%) h- (%)
benzene 89.41 0.563 2.55 2.67 67.19 25.01
toluene 106.85 0.590 2.59 1.83 60.17 21.92
mesilylene 139.58 0.532 0.86 0.98 40.15 13.88
~hl~~obenzene 102.24 0.605 2.81 2.31 105.52 31.02
shexane 131.61 0.501 1.22 1.06 6.94 5.75
wrbon tebachl~~lde 97.09 0.553 1.15 1.29 105.70 21.60
Tabla 2. Thermodynarnlc Data and Swelllng Parameters
1.0 - Molswlar
Solubility weight
0.8 - parameter Swelllng Volume Interaction between
6. (wl/ coefficient fraction parameter mass-links
Penetrant ~m~)''~ a *P X &.t
0.6 -
.
T
benzene
toluene
9.2
8.9
0.769
0.698
0.541
0.565
0.354
0.405
596
703
2-
0.4 - -
-
expCrimPnf
c ~ ~ c u l a t e from
d
masilylene
chlarobenzene
Mexane
8.8
9.5
7.3
0.463
0.958
0.106
0.662
0.467
0.896
0.456
0.340
1.416
600
890
614
carbon tetra- 6.6 0.666 0.576 0.473 710
0.2 - rhlnrida
used t o estimate the Flory-Huggins-type polymer-solvent
interaction parameter x as
Figure 3. ComDarisan of meOretlcal and experimental sorptlon curves for
polyurethane-Mexane system at 25 T.
where 6, and 6, represent the solubility parameters of liquid
and polymer, 6 is a lattice constant whose value is about 0.34,
agree quite satisfactorily. The concentration-independentD and Vs is the molar volume of the solvent, the term RT has
thus calculated is further used to generate the theoretical the usual meaning. For equilibrium swelling of a cross-
curve from eq 3; a typical plot is shown in Figure 3 for n- linked network in a solvent, the M, can be calculated using
hexane. The ohsemed good agreement between theoretical Flory and Rebner theory:
and exnerimental curves is sue~estiveof the reliahilitv of the
m e t h 2 of estimating D frogeither eq 4 or eq 6. F k h e r -
more, the exponent value n of eq 1, heing close to 0.5 but
varying u p to a maximum of 0.6, suggests that the diffusion
Drocess deviates sli,qhtly from the expected Fickian value where Q,, volume fraction of the polymer in the swollen
i n d could be classified as anomalous. membrane a t equilibrium with solvent, is calculated as
Several other useful ohsewations are ohtained from Fig-
ures 1 and 2. For instance, chlorobenzene shows highest
value for both Q(t) and h(t), whereas n-hexane shows the
lowest values. exhibitine almost a flat de~endenceof both The results of this analysis are given in Table 2. The value of
Q(f) and h(t)'on It ). ~ G p r i s i n ~forl ~carbon
, tetrachloride x indicates the strength of polymer-solvent interaction.
the Q ( 0 is hieher than those of the remainine Denetrants: on Generally, lower values of x (i.e., well below the assigned
the other hand, i t has a smaller h(t) than do benzene, tolu- value of x = %) indicates that the particular penetrant would
ene, or chlorobenzene. be a good solvent for the polymer in the absence of the
Further insight into polymer-solvent interactions may be restraining effects of hard segments of the polymer. In any
ohtained by the application of the Flory-Rehner model case. this method vields a somewhat satisfactorv Drocedure
(10.11) to the swelling data. This model assumes the depen- to estimate M, vilues, which are found to v&from one
dence of factors such as (1) molecular weiaht (M,) between Denetrant to the other.
cross-links, (2) volume fraction (Qp) of the polymer in its From the experimental result of Tables 1and 2 it is ohvi-
swollen state, and (3) polymer-solvent interaction parame- ous that high M, (106%) and h , (31%) as observed for the
ter (x) on the overall morphological behavior of the polymer. polyuretha~e-chlo~oben~ene system resulted in lower val-
In order to compute the M, values, we must obtain the ues of x (0.340) and higher values of M,(-890) as compared
sorption data in terms of swelling coefficient (a)defined as to the remaining liquids. This suggests that, out of all the
M-.-M, 1 monocyclic aromatics chosen for this investigation, chloro-
a=- (7)
M, PS
benzene is the good solvent. Similarly, carbon tetrachloride
behaves ina manner similar to that ofchlorobenzene.On the
where M, is the mass of the swollen polymer (i.e., equilibri- other hand, n-hexane, being a poor solvent for the polymer,
um saturation) and M, is the original mass of the polymer exhibited the smallest values for M , and h. and the highest
membrane; p, is the liquid density. These data are further value for x (1.416).
84 Journal of Chemical Education
Planning of Student Assignments lmpllcations and Conclusions
This experiment is somewhat time-consuming as it re- The study of transport of organic liquids through polv-
quires careful preparation by the student for a sequence of mers is important for a variety of engineering applicaiio"s.
experimental operations. We find that four to five continu- Contrary to gases, which diffuse through polymeric materi-
ous laboratory sessions of 4 hours each are required for a pair als with little interaction, the transpwt of liquids causes
of students 6 carry out duplicate determinations of D using polymers to swell; the extent of swelling depends upon ther-
both the experimental techniques. The entire experiment mal condition, chemical nature, and deeree of cross-linkine
can be completed in three days. We require the students to of the polymer in addition to the molar&ass of the
start from scratch-cutting the membrane to appropriate - - - and of the liquid molecule. I t is obvious that eq 6 suggests a
size, purifying and characterizing solvents, etc. new way of measuring D. This method is advantageous over
One of the virtues of this experiment is that i t provides the weight-gain technique in that the evaporation losses, if
different experimental and mathematical approaches to the any, do not affect the final results. However, such evapora-
same physical values. Effective educational use of the meth- tion losses are critical in the weight-gain method and affect
od and economy of the time can be achieved by assigning the final results. The agreement of diffusivity values as oh-
different pairs of students to different experiments. For tained by two entirely different methods is quite remark-
example, one pair of students can be assigned to one type of
liquid-polymer pair and evaluate D from both eqs 4 and 6.
able. I t is therefore concluded that the dimensional chanee
technique provides a very convenient method for measuring
-
The second, third, fourth, fifth, and sixth pairs of students the diffusivity of a solvent throueh a polvmer memhrane.
can be assigned the same determinations but using different Furthermoreithis technique is apilicabie to many polymer-
liquids for the same polymer. Another variation suggested is liquid systems whose p values are independent of the ab-
the determination of the same quantities over a ranee of sorbed penetrant; also, the experiments can be performed
temperatures. I t is desirable t h a t t h e values of diffusiiities with great precision and do not require any sophisticated
be obtained for a t least three temperatures. Such studies instrumentation.
lead to the evaluation of ~rrhenius-typeactivation parame-
ters involved in the transport processes. The three most Acknowledgment
convenient recommended temperatures for rubbery poly- We thank the University Grants Commission, New Delhi.
mers are 25,40, and 55 "C. for the award of teacher fellownhir, to USA under the Facul-
We find that followine such a nrocedure nroduces a ty Improvement Program.
healthy and stimulating interchange bf ideas ambng various Literature Cited
groups of students. At the end of the work. a session mav he 1. Jmt, W.Diffurion in Salids,Liquidsund Gares;Aeademie: New York, 1952.
Leldduring which results from various groups are presented 2. Crank, J. The Molhsmaficsaf Diffusion, 2nd ed.: Oxford University: ond don. 1975.
and compared. Thus, students will be exposed to "real- 3. Peppsu,N.A.;Urdhal,K.G.Eur.Polym.J. 1988,24,13.
4. 8mith.M. J.:Peppaa,N.A.Polymer1985.26.569.
world" and will develop skills necessary to bridge 5. Cahn, D.: Marom, G. Polym. Ens Sci. 1978,18,IW1.
from basic science to the solution of technological problems. 6. Cohn. D.; Msrom, G.Polymer 1979. W.501.
7. Cohn, D.; Merom, 0.Palym. En#.Sci. 1982,22,8A.
Afurther analvsis of the data in terms of suitihle theoretical
~ ~~ ~~ ~~~~~~
8. Aithal. U.S. PhDTheais, Kamatak University, 1989.
models, nameiy Flory-Rehner theory, leads to a better un- 9. Sanders,J. H.: F-h, K. C. Polyurethanes Chemistry and Technology, PLI, Chem.;
Krioger: New York. 1978.
derstanding of the polyrner-solvent interactions and gives 10. Flow, P.J. ??inciplssofPolymor Chemlalry: Cornell University: Ithaea, NY, 1953.
an insight into swelling mechanism. 11. Flow P. J.; Rehner, J., Jr. J. Chom.Phys.1943.11.521.
Call for Papers-Semon National Undergraduate Research Symposium
The 12th Annual Semon Lecture and National Undergraduate Research Symposium will be held at Kent State
University an Monday, April 2, 1990. This event is named in honor of Waldo Semon, who was the pioneer at the BF
Goodrich Company of Akron, OH, in the development of PVC and, after retirement, senredas a Research Professor at Kent
State University. A major focus of this event, which is co-sponsored by Kent State University and Goodrich, will be the
SemonNationalUndergraduateResearch Symposium. Students at colleges and universities anywhere in the United States
are invited to submit a paper describing their undergraduate research work. This should be limited to 10 double-spaced,
typed pages with any number of figures, tables, and references. Six finalists will be invited to present short seminars
describing their work at the symposium. The student presenting the best paper will receive a $2,000 prize, with $200 going
to each of the five remaining speakers. Limited travel funds will also be available. Interested students should write for
further details to: Paul Sampson, Chair, Semon Lecture Committee, Department of Chemistry, Kent State University,
Kent, OH 44242, or call (216) 672-2032. The deadline for receipt of papers is March 1,1990.
Volume 67 Number 1 January 1990 85
You might also like
- Interfacial Heat Transfer Coefficients of Various Vapors in Direct Contact CondensationDocument11 pagesInterfacial Heat Transfer Coefficients of Various Vapors in Direct Contact CondensationJesse Haney IIINo ratings yet
- MCQ For D - & F - Block ElementsDocument6 pagesMCQ For D - & F - Block ElementsAnshika Tripathi100% (3)
- Separator SizingDocument3 pagesSeparator SizingMaryJane Ayisha Sado-ObahNo ratings yet
- Design Calculation-Print A4Document51 pagesDesign Calculation-Print A4jne100% (1)
- Quality ControlDocument10 pagesQuality ControlB&R-QC KSPPLNo ratings yet
- LOT1 CONCRETE Mixes 27-03-2019 - REV 9.1Document7 pagesLOT1 CONCRETE Mixes 27-03-2019 - REV 9.1Soundar PachiappanNo ratings yet
- Adsorption of Acetic Acid with Activated CarbonDocument8 pagesAdsorption of Acetic Acid with Activated CarbonHayden Chappelear-RobbinsNo ratings yet
- Operating TablesDocument31 pagesOperating TablesJeffersonGutiérrez67% (3)
- Experiment 9Document6 pagesExperiment 9clairedemotica100% (1)
- Engineering DiagramsDocument19 pagesEngineering DiagramsLorenaNo ratings yet
- He Dressmaking Gr9 q1 Module-2Document23 pagesHe Dressmaking Gr9 q1 Module-2reymilyn zuluetaNo ratings yet
- Determination of Diffusion and Mass Transfer Coefficients During Drying of Solvent-Absorbed Polymer FilmsDocument7 pagesDetermination of Diffusion and Mass Transfer Coefficients During Drying of Solvent-Absorbed Polymer FilmsSiraj AL sharifNo ratings yet
- A Mathematical Model For Dispersion in The Direction of Flow in Porous MediaDocument4 pagesA Mathematical Model For Dispersion in The Direction of Flow in Porous MediaBahman MatouriNo ratings yet
- Gonzales Nonionik SurfaktanDocument6 pagesGonzales Nonionik SurfaktanMathilda Jowito PasaribuNo ratings yet
- Theoretical and experimental studies on acetylene absorptionDocument9 pagesTheoretical and experimental studies on acetylene absorptionJames Laurence RavizNo ratings yet
- J. Wijmans - R. Baker - The Solution Diffusion Model - A ReviewDocument21 pagesJ. Wijmans - R. Baker - The Solution Diffusion Model - A ReviewNicolasNo ratings yet
- Kinetic Modeling of Liquid-Phase Adsorption of Reactive Dyes On Activated CarbonDocument10 pagesKinetic Modeling of Liquid-Phase Adsorption of Reactive Dyes On Activated CarbonTobias De SomerNo ratings yet
- Theory of Transport in MembranesDocument17 pagesTheory of Transport in MembranesMuhammad Fattah Romdhoni100% (1)
- CE 480 - Membrane Processes - 02Document24 pagesCE 480 - Membrane Processes - 02NTEYE CHITONGENo ratings yet
- Asmussen 1962Document10 pagesAsmussen 1962mr.snorhaarNo ratings yet
- Protein Ion-Exchange Adsorption KineticsDocument10 pagesProtein Ion-Exchange Adsorption KineticsJordan Hiles-BrownNo ratings yet
- Ticknor 1958Document3 pagesTicknor 1958Juanda DariusNo ratings yet
- Indentation Creep of Polymeric Materials: Experimental and AnalysisDocument6 pagesIndentation Creep of Polymeric Materials: Experimental and Analysismohsen shabanloNo ratings yet
- A Comparison Between The Different Methods For The Measurement of An Excess Adsorption of Pure Gases On Porous Adsorbents at High PressureDocument8 pagesA Comparison Between The Different Methods For The Measurement of An Excess Adsorption of Pure Gases On Porous Adsorbents at High PressureHaseeb JatoiNo ratings yet
- A Discrete Model For The Apparent Viscosity of Polydisperse Suspensions Including Maximum Packing FractionDocument14 pagesA Discrete Model For The Apparent Viscosity of Polydisperse Suspensions Including Maximum Packing FractionLuke ParryNo ratings yet
- Suspensions Through Porous: MedidDocument28 pagesSuspensions Through Porous: MedidReza KazemiNo ratings yet
- MembranesDocument30 pagesMembranesInamullah MaitloNo ratings yet
- MembranesDocument30 pagesMembranesmiraziey100% (1)
- Artículo Liquid Phase Mass Transfer Resistancec in Small Scale Packed Distillation CollumnDocument7 pagesArtículo Liquid Phase Mass Transfer Resistancec in Small Scale Packed Distillation CollumnAnaid GarciaNo ratings yet
- A Methodology To Derive The Seepage Law of Power-Law Fluids Through Fibrous MediaDocument7 pagesA Methodology To Derive The Seepage Law of Power-Law Fluids Through Fibrous MediaUmed Abd-alsatarNo ratings yet
- 00 Yang ChemEngTechnolDocument6 pages00 Yang ChemEngTechnollabichhuongNo ratings yet
- Composite PDMSDocument11 pagesComposite PDMSSimon ChovauNo ratings yet
- 1966 American Institute of Mining, Nletrdlrrrgical and Petroleum Engineers, IncDocument4 pages1966 American Institute of Mining, Nletrdlrrrgical and Petroleum Engineers, IncWaleed Barakat MariaNo ratings yet
- Di8Nsion A-Iron : Coefbcient of C inDocument5 pagesDi8Nsion A-Iron : Coefbcient of C inMahsaNo ratings yet
- Capillary Breakup Extensional Rheometry of Semi-Dilute Polymer SolutionsDocument9 pagesCapillary Breakup Extensional Rheometry of Semi-Dilute Polymer SolutionsDuong MaiNo ratings yet
- pak2008Document7 pagespak2008Naeem owaisNo ratings yet
- Models For Mass Transfer CoefficientDocument2 pagesModels For Mass Transfer Coefficientlopiga21203827mNo ratings yet
- Numerical Simulation of Toluene Vapor Diffusion in Lim - 2014 - Procedia EngineeDocument6 pagesNumerical Simulation of Toluene Vapor Diffusion in Lim - 2014 - Procedia EngineejanainaNo ratings yet
- Al Khayat2016Document9 pagesAl Khayat2016Hanh DuongNo ratings yet
- Control de La Reologia Utlizando AsociativoDocument11 pagesControl de La Reologia Utlizando AsociativoLATINA DE PINTURASNo ratings yet
- OdenbrandDocument7 pagesOdenbrandAndré BassiNo ratings yet
- C8 Cussler PDFDocument33 pagesC8 Cussler PDFRaisa LopezNo ratings yet
- Macromolecular Microsymposium — 16: Main Lectures Presented at the Sixteenth Microsymposium on Macromolecules (Advances in Scattering Methods), Prague, 12 - 16 July 1976From EverandMacromolecular Microsymposium — 16: Main Lectures Presented at the Sixteenth Microsymposium on Macromolecules (Advances in Scattering Methods), Prague, 12 - 16 July 1976B. SedláčekNo ratings yet
- Two Methods For Determination of Transport Numbers in Ion Exchange MembranesDocument19 pagesTwo Methods For Determination of Transport Numbers in Ion Exchange MembranesLuis AlvarezNo ratings yet
- Hemodialyzer ClearanceDocument3 pagesHemodialyzer ClearanceMohammadReza MoradiNo ratings yet
- 2005 Effects of Petrophysical Rock Properties On Tortuosity Factor - Attia M. Attia PDFDocument14 pages2005 Effects of Petrophysical Rock Properties On Tortuosity Factor - Attia M. Attia PDFHafizhan Abidin SetyowiyotoNo ratings yet
- Jresv67an6p615 A1bDocument10 pagesJresv67an6p615 A1bterryphiNo ratings yet
- FQII-Article 1 - TASCA BLOC IDocument4 pagesFQII-Article 1 - TASCA BLOC ICristina Enrich TorrentsNo ratings yet
- Convection Diffusion and Reaction InsideDocument11 pagesConvection Diffusion and Reaction InsideHarsh TekriwalNo ratings yet
- JMemSc254 (2005) 267Document8 pagesJMemSc254 (2005) 267api-26678889No ratings yet
- Effect of Adsorption on Lumped Rate Coefficients of ProteinsDocument6 pagesEffect of Adsorption on Lumped Rate Coefficients of ProteinsCelso Hissao MaedaNo ratings yet
- Experiment 6Document20 pagesExperiment 6Saniha Aysha AjithNo ratings yet
- PhysFluids 17 058101Document4 pagesPhysFluids 17 058101Omid EjtehadiNo ratings yet
- Playing With Liquid Foams: Learning Physical ChemistryDocument3 pagesPlaying With Liquid Foams: Learning Physical Chemistrymeera4arunNo ratings yet
- The Diffusion Time Lag in Polymer Membranes Containing Adsorptive FillersDocument15 pagesThe Diffusion Time Lag in Polymer Membranes Containing Adsorptive FillersnimzaiNo ratings yet
- Lake and Hirasaki (1981) (SPE-8436-PA)Document10 pagesLake and Hirasaki (1981) (SPE-8436-PA)Anonymous PO7VwbBnNo ratings yet
- Transport Through Polymer Membranes: G The Polymer Is in Its GlassyDocument8 pagesTransport Through Polymer Membranes: G The Polymer Is in Its Glassyeni_cristianNo ratings yet
- PhysRevE 54 406Document5 pagesPhysRevE 54 406ASHES BANERJEENo ratings yet
- Fundamental Principles of Ultrafiltration PDFDocument15 pagesFundamental Principles of Ultrafiltration PDFalmutaz9879No ratings yet
- To Lagrangian Models of Particle The Atmospheric YerDocument12 pagesTo Lagrangian Models of Particle The Atmospheric YerAida Nur FadhilahNo ratings yet
- Pore Pressure Transients and The Advective Transport ProblemDocument7 pagesPore Pressure Transients and The Advective Transport ProblemUmed Abd-alsatarNo ratings yet
- The Range of Validity of Graham's LawDocument6 pagesThe Range of Validity of Graham's LawJulio Carlos De La Paz CruzNo ratings yet
- Filtration of Micropolar Liquid Through A Membrane Composed of Spherical Cells With Porous Layer D. Yu. KhanukaevaDocument31 pagesFiltration of Micropolar Liquid Through A Membrane Composed of Spherical Cells With Porous Layer D. Yu. KhanukaevaRoberticoZeaNo ratings yet
- Dixon PaperDocument6 pagesDixon Paperyesol wooNo ratings yet
- High Resolution X-Ray and Light Scattering Studies of Bilayer Smectic A CompoundsDocument6 pagesHigh Resolution X-Ray and Light Scattering Studies of Bilayer Smectic A CompoundsFernando Garcia GoldingNo ratings yet
- Ardell2020 - Article - Trans Interface Diffusion ContDocument23 pagesArdell2020 - Article - Trans Interface Diffusion Contkandula.munikumarNo ratings yet
- A Theory of Constant Pressure FiltrationDocument12 pagesA Theory of Constant Pressure FiltrationLuis Alfonso Galvan MoralesNo ratings yet
- REE IffusionDocument38 pagesREE IffusionLeillane BeatrizNo ratings yet
- Austin LJ 1966 PHD ThesisDocument245 pagesAustin LJ 1966 PHD ThesisLorenaNo ratings yet
- Practica ViscosityDocument7 pagesPractica ViscosityLorenaNo ratings yet
- 2-Ethylanthraquinone: Safety Data SheetDocument4 pages2-Ethylanthraquinone: Safety Data SheetLorenaNo ratings yet
- Solvents SDocument3 pagesSolvents SLorenaNo ratings yet
- Solvents SDocument3 pagesSolvents SLorenaNo ratings yet
- Sigma-Aldrich: Safety Data SheetDocument7 pagesSigma-Aldrich: Safety Data SheetLorenaNo ratings yet
- Types of Bonding, Callister and Rethwisch Chapter 2: Homework #1Document1 pageTypes of Bonding, Callister and Rethwisch Chapter 2: Homework #1LorenaNo ratings yet
- Workshop 1Document8 pagesWorkshop 1LorenaNo ratings yet
- Infografía (Diagrama de Bloques)Document1 pageInfografía (Diagrama de Bloques)LorenaNo ratings yet
- Boiler Operation and Safety GuideDocument19 pagesBoiler Operation and Safety GuidemarlpatsNo ratings yet
- Plasticity in Structural Engineering Fundamentals and ApplicationsDocument10 pagesPlasticity in Structural Engineering Fundamentals and Applicationstemp ovaryNo ratings yet
- Heat TransferDocument27 pagesHeat TransferOmar EzzatNo ratings yet
- Thermal Engineering for 500 MW BoilerDocument31 pagesThermal Engineering for 500 MW BoilerRituraaj Singh RajputNo ratings yet
- Pegamento para TuberiaDocument4 pagesPegamento para TuberiaJesus RoaNo ratings yet
- 3.3 BleachingDocument28 pages3.3 Bleachingshym hjrNo ratings yet
- Lab Ledger PhyDocument28 pagesLab Ledger PhyMumtazAhmadNo ratings yet
- 02 Quote CIF GBM Remote Control GrabDocument7 pages02 Quote CIF GBM Remote Control GrabPanggi Eko PrasetiyoNo ratings yet
- 2023 CHEMISTRY F3 P2 QS T2 Exam Teacher - Co - .KeDocument9 pages2023 CHEMISTRY F3 P2 QS T2 Exam Teacher - Co - .Kealooben2No ratings yet
- Low Density Polyethylene: DescriptionDocument2 pagesLow Density Polyethylene: DescriptionlyesNo ratings yet
- Comparative Study of Design of Water Tank With Reference To Is: 3370Document4 pagesComparative Study of Design of Water Tank With Reference To Is: 3370Rahul KolateNo ratings yet
- Fatai Kolawole Ikumapayi Lic2010Document182 pagesFatai Kolawole Ikumapayi Lic2010Gaurav MeshramNo ratings yet
- Polyglass: Page 1 of 2Document2 pagesPolyglass: Page 1 of 2whyme_bNo ratings yet
- Saej402v002 PDFDocument8 pagesSaej402v002 PDFLuis LujanoNo ratings yet
- Kcse 2023 Joint Mocks s1Document265 pagesKcse 2023 Joint Mocks s1micah isabokeNo ratings yet
- 1 s2.0 S1359431123015909 MainDocument33 pages1 s2.0 S1359431123015909 Mainfabio1199No ratings yet
- Piping Materials Match Chart (ASTM) - ProjectmaterialsDocument14 pagesPiping Materials Match Chart (ASTM) - ProjectmaterialsdhurjatibhuteshNo ratings yet
- SKF - Large Diameter Seals - 6404 EngDocument116 pagesSKF - Large Diameter Seals - 6404 EngDiegoAlvarezHuguezNo ratings yet
- Capillary Processes in Porous Media: An Introduction to Soil PhysicsDocument65 pagesCapillary Processes in Porous Media: An Introduction to Soil Physics노경보No ratings yet
- SDS - Chemrock 958aDocument1 pageSDS - Chemrock 958amangengueyNo ratings yet
- Conductive Plastics For Electrical and Electronic Applications PDFDocument4 pagesConductive Plastics For Electrical and Electronic Applications PDFsonchemenNo ratings yet