Professional Documents
Culture Documents
Session 8 Topic Free Energy Composition Curves For Binary Systems
Uploaded by
Thaya GanapathyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Session 8 Topic Free Energy Composition Curves For Binary Systems
Uploaded by
Thaya GanapathyCopyright:
Available Formats
FREE ENERGY COMPOSITION CURVES FOR BINARY SYSTEMS
Free energy composition curves for binary systems
Consider the Gibbs free energy of one mole of atoms (molar gibbs free energy), some
of which are A atoms and some of which are B atoms. Consider G(XB), that is, resultant ‘G’
as we change the relative amounts of A and B.
SNSCE /S & H / 2nd Semester / PH8251 - Materials Science Page 1
SNS COLLEGE OF ENGINEERING, Coimbatore
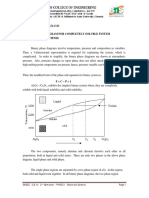
If the (molar) Gibbs free energy of pure A is GA, and that of pure B is GB, then the
(molar) Gibbs free energy for the combination of pure components is
G(pure, combined) = GA•XA+ GB•XB
As a function of composition, the Gibbs free energy for the combination of pure A
and pure B is a straight line connecting GA and GB, as shown below:
Now, let us remove the imaginary partition and let the A and B atoms mix. There
should be some change in g due to this mixing, and it is given by
∆Gmix = ∆Hmix - T∆Smix
The enthalpy term,
∆Hmix, represents the nature of the chemical bonding, or, put in different words, the extent to
which A prefers B, or A prefers A as a neighbor. The entropy term, ∆Smix, signifies the
increase in disorder in the system as we let the A and B atoms mix. It is independent of the
nature of the chemical bonding. We can approximate our isomorphous, binary system as an
ideal solution, therefore
∆Hmix = 0.
Let us qualitatively examine the entropy of mixing. If we have a system of pure A,
and let the A atoms “mix” with one another, there is no increase in entropy because they were
mixed to begin with. The same holds true for a system of pure B. If we have just one atom of
B in a mole of A, removing the invisible partition hardly changes the amount of disorder.
But, as we get to a 50:50 composition of A:B, the increase in entropy as we allow the system
to mix is enormous. Therefore, ∆Smix has the following dependence on composition.
SNSCE /S & H / 2nd Semester / PH8251 - Materials Science Page 2
SNS COLLEGE OF ENGINEERING, Coimbatore
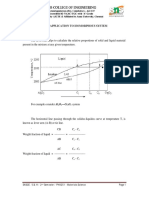
From that we can easily get the change in Gibbs free energy due to mixing:
Now we sum G(pure, combined) and ∆Gmix to get the total Gibbs free energy of the
solution as a function of composition:
At this point we have the general shape of the G(XB) curves for phases in a binary
system. This overall shape holds for both the solid and liquid phases, so long as both make
ideal (or close to ideal) solutions.
SNSCE /S & H / 2nd Semester / PH8251 - Materials Science Page 3
You might also like
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Binary Phase Diagrams: Binary Phase Diagram For Completely Soluble System (Isomorphous Systems)Document2 pagesBinary Phase Diagrams: Binary Phase Diagram For Completely Soluble System (Isomorphous Systems)Thaya GanapathyNo ratings yet
- PH8251-Material Science QN Bank ValliammaiDocument10 pagesPH8251-Material Science QN Bank ValliammaiThaya GanapathyNo ratings yet
- Session 5 Topic 5 Isomorphous Systems, The Tie-Line RuleDocument2 pagesSession 5 Topic 5 Isomorphous Systems, The Tie-Line RuleThaya GanapathyNo ratings yet
- Microstructure of A Lead-Tin AlloyDocument55 pagesMicrostructure of A Lead-Tin AlloyThaya GanapathyNo ratings yet
- Modern Engineering Materials: Unit - VDocument11 pagesModern Engineering Materials: Unit - VThaya GanapathyNo ratings yet
- Unit Iii Thermal Physics: Department of Science and Humanities MCQ For Regulations 2017Document19 pagesUnit Iii Thermal Physics: Department of Science and Humanities MCQ For Regulations 2017Thaya GanapathyNo ratings yet
- Unit I MCQDocument18 pagesUnit I MCQThaya GanapathyNo ratings yet
- Physics For Bioscience: Rajalakshmi Engineering College (Autonomous) Thandalam, Chennai - 602105Document193 pagesPhysics For Bioscience: Rajalakshmi Engineering College (Autonomous) Thandalam, Chennai - 602105Thaya GanapathyNo ratings yet
- Topic 2.1 Oscillatory Motion: Department of Science and Humanities MCQ For Regulations 2017Document13 pagesTopic 2.1 Oscillatory Motion: Department of Science and Humanities MCQ For Regulations 2017Thaya GanapathyNo ratings yet
- Question Bank US03CPHY01 Unit1 To 4 Optics PMPDocument13 pagesQuestion Bank US03CPHY01 Unit1 To 4 Optics PMPThaya GanapathyNo ratings yet
- Exam1 SolutionsDocument4 pagesExam1 SolutionsThaya GanapathyNo ratings yet
- 08 - Chapter 1Document19 pages08 - Chapter 1Thaya GanapathyNo ratings yet
- Solid State Physics 1 PGDocument4 pagesSolid State Physics 1 PGThaya GanapathyNo ratings yet
- 10 - Chapter 1Document24 pages10 - Chapter 1Thaya GanapathyNo ratings yet
- Electricity and MagnetismDocument275 pagesElectricity and MagnetismThaya Ganapathy100% (3)
- 11 Fluids 1Document13 pages11 Fluids 1Thaya GanapathyNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Materials Engineers Review Notes Set FourDocument3 pagesMaterials Engineers Review Notes Set Foursantiago andresNo ratings yet
- Adhimix Trial Mix DocumentationDocument92 pagesAdhimix Trial Mix Documentationyudha satriaNo ratings yet
- Abstract Sobolewski Coking Coal To CokeDocument27 pagesAbstract Sobolewski Coking Coal To CokeFilipe Mansur100% (1)
- Turbine Oil Condition Monitoring Training GuideDocument8 pagesTurbine Oil Condition Monitoring Training Guidemauriciojj100% (1)
- DHP Product Information Sheet EXPAND O LIZER BSDocument2 pagesDHP Product Information Sheet EXPAND O LIZER BSyelena-sNo ratings yet
- Steel Fabrication Method StatementDocument12 pagesSteel Fabrication Method Statementvolcanox1288% (59)
- 111column DesignDocument16 pages111column DesignAbdirahman Ahmed IsseNo ratings yet
- BC Gluelam Guide PDFDocument36 pagesBC Gluelam Guide PDFklb75100% (1)
- Cambridge IGCSE: CHEMISTRY 0620/62Document12 pagesCambridge IGCSE: CHEMISTRY 0620/62ShehabNo ratings yet
- Refractories For Steel MakingDocument36 pagesRefractories For Steel MakingradinasrNo ratings yet
- GROUP3 Hand Outs Voltage Current and ResistanceDocument7 pagesGROUP3 Hand Outs Voltage Current and Resistancekristel castroNo ratings yet
- Modern Theories of Acids & Bases: The Arrhenius and Bronsted-Lowry TheoriesDocument48 pagesModern Theories of Acids & Bases: The Arrhenius and Bronsted-Lowry TheoriesAgung PratamaNo ratings yet
- Quimica Del GalioDocument44 pagesQuimica Del Galiomglez2012No ratings yet
- BiocompostingDocument54 pagesBiocompostingPAMELA100% (1)
- Mechanical Properties of NiTi and CuNiTi Shape-Memory Wires Used in Orthodontic Treatment. Part 1: Stress-Strain TestsDocument9 pagesMechanical Properties of NiTi and CuNiTi Shape-Memory Wires Used in Orthodontic Treatment. Part 1: Stress-Strain TestsClaudiaNo ratings yet
- Structural Crack StudyDocument9 pagesStructural Crack StudyNurmuliana Abdul WahabNo ratings yet
- CHM 3201 - Lab #1 The Prep. of Copper 1 Chloride WRDocument12 pagesCHM 3201 - Lab #1 The Prep. of Copper 1 Chloride WRRaja GokhulNo ratings yet
- Reviewer in Inorganic ChemistryDocument5 pagesReviewer in Inorganic ChemistryPrincess Aleia SalvadorNo ratings yet
- Matt80 HM100 WG62Document2 pagesMatt80 HM100 WG62Mahmud RezaNo ratings yet
- HydroPlus Information Website PDFDocument1 pageHydroPlus Information Website PDFTere CastellanosNo ratings yet
- LIC & ApplicationsDocument92 pagesLIC & ApplicationsAbhiroop VermaNo ratings yet
- Aerosil R812 SDocument6 pagesAerosil R812 SRoxanaNo ratings yet
- Mercury PresentationDocument42 pagesMercury Presentationpejal5284100% (5)
- Method Statement For Construction of PSC GIRDERDocument2 pagesMethod Statement For Construction of PSC GIRDERAsad AshfaqNo ratings yet
- Api 653Document1 pageApi 653BashMohandesssNo ratings yet
- Metal Air Battery - An Overview - ScienceDirect TopicsDocument10 pagesMetal Air Battery - An Overview - ScienceDirect TopicsSenen100% (1)
- Book Catalogue 2021Document79 pagesBook Catalogue 2021navecNo ratings yet
- 3 E Recycled Glass As A Partial Replacement For Fine Aggregate in Self Compacting ConcreteDocument7 pages3 E Recycled Glass As A Partial Replacement For Fine Aggregate in Self Compacting Concretemaverick ownadorNo ratings yet
- Steel Metallurgy PDFDocument511 pagesSteel Metallurgy PDFTiên TrầnNo ratings yet
- Diamond Wire CuttingDocument11 pagesDiamond Wire CuttingRafael Dos SantosNo ratings yet