Professional Documents
Culture Documents
Foundation Chemistry CHEM 125 Module 1: The Periodic Table Assignment 1
Uploaded by
METAL LEEOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Foundation Chemistry CHEM 125 Module 1: The Periodic Table Assignment 1
Uploaded by
METAL LEECopyright:
Available Formats
Foundation Chemistry
CHEM 125
Module 1: The Periodic Table
Assignment 1
Instructions:
This assignment consists of ten questions. Each question is worth 1 mark each. Answer all
questions on a separate sheet. You do not have to retype the questions. You must however, ensure
that each answer is placed next to the corresponding question number.
Remember, chemistry is ‘case sensitive’ therefore you must carefully distinguish between
uppercase and lower case (eg. The symbol for calcium is Ca not ca or CA). You must also carefully
distinguish between superscript (eg. O2-) and subscript (O2).
Use the periodic table where necessary.
Submission specifications:
1. Answers must be type written.
2. Your answer sheet MUST be saved as a pdf document and uploaded/submitted before
11:50pm on Friday 17th September, 2021.
Questions
1) Which of the following is NOT a compound?
a) NaCl b) Na c) KBr d) LiF
2) Which of the following is NOT a type of radiation discovered by Ernest Rutherford?
a) delta (δ) b) alpha (α) c) beta (β) d) gamma (γ)
3) Which of the following statements is correct?
a) Electrons have a positive charge (1+) and a mass of 1.00amu.
b) Protons have a positive charge (1+) and a mass of 1.00amu.
c) Neutrons have a negative charge (1-) and a mass of 1.00amu.
d) Nucleus have a neutral charge (0) and a mass of 5.49 x 10 -4 amu.
4) Which element in the periodic table is represented by the symbol P?
a) potassium b) palladium c) phosphorus d) platinum
5) What is the electron configuration of nitrogen?
a) 1s2 2p7 b) 1s7 2p14 c) 1s2 2p14 d) 1s2 2p5
6) What is the name of the element that has 20 protons in the nucleus of its atoms?
a) sodium b) neon c) copper d) calcium
7) The table below describes four atoms.
Atom A Atom B Atom C Atom D
Number of protons 10 11 11 10
Number of neutrons 11 10 11 10
Number of electrons 10 11 11 10
Are atoms A and C isotopes? Answer yes or no.
8) Identify the metal from among the following elements.
a) K b) C c) O d) Br
9) How many electrons are in the valence shells of elements in group 2A of the periodic table?
a) 2 b) 1 c) 6 d) 8
10) Which of the following atoms has the highest electronegativity?
a) P b) F c) Li d) K
You might also like
- Chemistry The Molecular Nature of Matter and Change 7th Edition Silberberg Test BankDocument16 pagesChemistry The Molecular Nature of Matter and Change 7th Edition Silberberg Test Banka4645830560% (1)
- General ChemistryDocument27 pagesGeneral ChemistryRick AndrewsNo ratings yet
- Business PlanDocument22 pagesBusiness PlanRanjitha Kalaiselvan100% (2)
- Tubular & Non Tubular AWS D1.1Document56 pagesTubular & Non Tubular AWS D1.1Rico Ferdian100% (1)
- Fpso - LNG ProcessDocument15 pagesFpso - LNG ProcessYeshWaNth100% (1)
- Chemistry Chang 11th Edition Test Bank Full DownloadDocument18 pagesChemistry Chang 11th Edition Test Bank Full Downloadkatherineguzmanqrzncbmida100% (32)
- Grade 9Document3 pagesGrade 9letty louNo ratings yet
- Science Stage 8 Sample Paper 1 - tcm143-595703Document15 pagesScience Stage 8 Sample Paper 1 - tcm143-595703Joseph Jerry-Oche100% (2)
- Dwnload Full Introductory Chemistry For Today 8th Edition Seager Test Bank PDFDocument6 pagesDwnload Full Introductory Chemistry For Today 8th Edition Seager Test Bank PDFschmitzerallanafx100% (11)
- 2.02 Chemistry Intro Quiz (G9 Review) 2020-2021Document3 pages2.02 Chemistry Intro Quiz (G9 Review) 2020-2021ocNo ratings yet
- Page Number: 81: Subject-Chemistry DATE - 22/12/2020 Class - X TOPICS - Solutions of Textual Questions of Chapter 5Document5 pagesPage Number: 81: Subject-Chemistry DATE - 22/12/2020 Class - X TOPICS - Solutions of Textual Questions of Chapter 5Umar Aman VirkNo ratings yet
- Chemistry For Today General Organic and Biochemistry 8th Edition Seager Test BankDocument36 pagesChemistry For Today General Organic and Biochemistry 8th Edition Seager Test Bankalborakinfect.ufid12100% (27)
- CBSE Class 10 Science Chapter 5 NCERT Solutions 2022 - Free PDFDocument7 pagesCBSE Class 10 Science Chapter 5 NCERT Solutions 2022 - Free PDFMuzafar ahmadNo ratings yet
- Lichter CHM1045 Quizzes (8A) (Spring 2012)Document8 pagesLichter CHM1045 Quizzes (8A) (Spring 2012)Jules BrunoNo ratings yet
- Chemistry - Textbook Answers Chapter 5Document20 pagesChemistry - Textbook Answers Chapter 5angelina_boseNo ratings yet
- Gr9 OSSD Chemistry RevisionDocument4 pagesGr9 OSSD Chemistry RevisionocNo ratings yet
- Periodic Classification of Elements: Chapter-5Document98 pagesPeriodic Classification of Elements: Chapter-5Throwaway AccountNo ratings yet
- Screenshot 2023-07-27 at 12.06.37 PMDocument2 pagesScreenshot 2023-07-27 at 12.06.37 PMKiyoshi RenNo ratings yet
- Science 9 Quiz# 1 2nd QDocument3 pagesScience 9 Quiz# 1 2nd QEmmanuel LlonaNo ratings yet
- Chemistry For Today General Organic and Biochemistry 8th Edition Seager Test BankDocument26 pagesChemistry For Today General Organic and Biochemistry 8th Edition Seager Test BankSharonVargasgjme100% (59)
- Examination 4: Multiple Choice QuestionsDocument7 pagesExamination 4: Multiple Choice QuestionsMohamad Idris SaidinNo ratings yet
- Exercise Solution of Periodic ElementsDocument9 pagesExercise Solution of Periodic ElementsiTutor Classes BapiNo ratings yet
- 10 Science TP 5 1Document5 pages10 Science TP 5 1Nawaab PuneetNo ratings yet
- CBSE Class 9 Science Chapter 4 Structure of The Atom Important Questions 2022-23Document20 pagesCBSE Class 9 Science Chapter 4 Structure of The Atom Important Questions 2022-23Mohammed Javed KhanNo ratings yet
- Conceptual Chemistry 5Th Edition Suchocki Test Bank Full Chapter PDFDocument36 pagesConceptual Chemistry 5Th Edition Suchocki Test Bank Full Chapter PDFsuzanne.guillory241100% (9)
- Chapter 5 Periodic Classification of ElementsDocument9 pagesChapter 5 Periodic Classification of ElementsasuhassNo ratings yet
- U2 Exam PRACTICEDocument7 pagesU2 Exam PRACTICEAlexis61No ratings yet
- ICSE Class 8 Chemistry Selina Solution Chapter 4 Atomic StructureDocument8 pagesICSE Class 8 Chemistry Selina Solution Chapter 4 Atomic StructureAmmolh MahajanNo ratings yet
- Cbse 2020 Boards MCQ ScienceDocument5 pagesCbse 2020 Boards MCQ ScienceAbuzar AzharNo ratings yet
- Prelims Ans Key ChemDocument5 pagesPrelims Ans Key ChemZahra SaifyNo ratings yet
- 0e729488 02b4 47e5 A21d E7ca032be3d8 - Revision Sheet 2 Answer KeyDocument8 pages0e729488 02b4 47e5 A21d E7ca032be3d8 - Revision Sheet 2 Answer KeySharon BijuNo ratings yet
- Practice Questions For Quiz 1Document7 pagesPractice Questions For Quiz 1TkNo ratings yet
- Subject: Chemistry: Gcse Higher Tier Topic Test SeriesDocument7 pagesSubject: Chemistry: Gcse Higher Tier Topic Test SeriesKakoli PaulNo ratings yet
- Building Blocks of MatterDocument33 pagesBuilding Blocks of MatterSamantha TomistaNo ratings yet
- Chemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Test BankDocument36 pagesChemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Test Bankalborakinfect.ufid12100% (24)
- Unit 1 - Test 1 - AOLDocument9 pagesUnit 1 - Test 1 - AOLRayyan SadruddinNo ratings yet
- Che1031 Quiz 2 KeyDocument3 pagesChe1031 Quiz 2 KeykdNo ratings yet
- Homework Packet / Unit 2: HW #2.1: Contained in Separate Handout HW #2.2Document7 pagesHomework Packet / Unit 2: HW #2.1: Contained in Separate Handout HW #2.2api-368121935No ratings yet
- (A) Atomic Number (C) Att Mic Volume (D) NoneDocument3 pages(A) Atomic Number (C) Att Mic Volume (D) NoneThe Noor SchoolNo ratings yet
- Icse Class 7 Worksheet 7 PDFDocument16 pagesIcse Class 7 Worksheet 7 PDFNABHAN CHOPRANo ratings yet
- Structure of The Atom Class IxDocument3 pagesStructure of The Atom Class Ixfarooquima5327No ratings yet
- CH 05Document30 pagesCH 05ffffffff dfdfdfNo ratings yet
- CHEM1 Model MCQ PDFDocument13 pagesCHEM1 Model MCQ PDFMiku HatsuneNo ratings yet
- Test Bank For Biology Life On Earth With Physiology 10Th Edition Audesirk Byers Isbn 0321794265 9780321794260 Full Chapter PDFDocument36 pagesTest Bank For Biology Life On Earth With Physiology 10Th Edition Audesirk Byers Isbn 0321794265 9780321794260 Full Chapter PDFmaria.rodriguez942100% (11)
- Topic 3 Atomic Structure AnswersDocument13 pagesTopic 3 Atomic Structure AnswersKaixin HuangNo ratings yet
- Structure of Atom For Class 9 Solved Summative AssesmentDocument23 pagesStructure of Atom For Class 9 Solved Summative AssesmentSabu VincentNo ratings yet
- Perfect Tutorial: Sub-Chemistry Class - XDocument3 pagesPerfect Tutorial: Sub-Chemistry Class - Xvineetvishal73No ratings yet
- Test Bank For Conceptual Chemistry 4th Edition SuchockiDocument40 pagesTest Bank For Conceptual Chemistry 4th Edition Suchockipatricklongrwfmzkjotd100% (26)
- Problem Set 1 Rev 1Document3 pagesProblem Set 1 Rev 1edelyn telewikNo ratings yet
- KeyDocument5 pagesKeyKali corgiNo ratings yet
- NCERT CBSE Solutions For Class 10 Science Chapter 5: Periodic Classification of ElementsDocument8 pagesNCERT CBSE Solutions For Class 10 Science Chapter 5: Periodic Classification of ElementsASIFNo ratings yet
- Chapter 8: Periodic Relationships Among The ElementsDocument14 pagesChapter 8: Periodic Relationships Among The Elements216435964No ratings yet
- Atomic StructureDocument27 pagesAtomic StructureBiswajit SwainNo ratings yet
- Classification of Element ch-3 TestDocument4 pagesClassification of Element ch-3 TestUtkarsh kumarNo ratings yet
- CBSE Class 9 Science Worksheet - Structure of AtomDocument2 pagesCBSE Class 9 Science Worksheet - Structure of AtomAjeetNo ratings yet
- P4 Atomic Structure Combined ScienceDocument42 pagesP4 Atomic Structure Combined Sciencesamibarcelona08No ratings yet
- CLASS 9 CHEMISTRY BenevolenceDocument3 pagesCLASS 9 CHEMISTRY BenevolenceFARHAN KAMALNo ratings yet
- Prelim Exam Review Chemistry For Engineers FinalDocument102 pagesPrelim Exam Review Chemistry For Engineers Finalkat rinaNo ratings yet
- Chapter#1: Choose The Best AnswerDocument2 pagesChapter#1: Choose The Best AnswerMohammad AshfaqNo ratings yet
- Online Assessment Test (Ii) 2021-2022: Blessed Sacrament High School, Puri Std-Ix Chemistry FM-100Document3 pagesOnline Assessment Test (Ii) 2021-2022: Blessed Sacrament High School, Puri Std-Ix Chemistry FM-100KPS SHREYASNo ratings yet
- Unit 1 Practice MC 2021 Directions: Choose The Best Answer, Then Check The Answer KEY at The EndDocument5 pagesUnit 1 Practice MC 2021 Directions: Choose The Best Answer, Then Check The Answer KEY at The EndActiveNo ratings yet
- The Elite Test Session 2017 Chemistry: 40 Chapter # 1Document1 pageThe Elite Test Session 2017 Chemistry: 40 Chapter # 1Tanveer AkramNo ratings yet
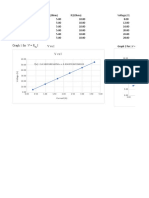
- Vvsi Vvsi: Vvsi Graph 1 For:V R IDocument2 pagesVvsi Vvsi: Vvsi Graph 1 For:V R IMETAL LEENo ratings yet
- Leyhanessa Rolle Revised Comparative EssayDocument6 pagesLeyhanessa Rolle Revised Comparative EssayMETAL LEENo ratings yet
- APA Format and Citation Exercise Fall 2021Document4 pagesAPA Format and Citation Exercise Fall 2021METAL LEENo ratings yet
- 2021 Lab 4 Leaf Surface Area LabDocument5 pages2021 Lab 4 Leaf Surface Area LabMETAL LEENo ratings yet
- FERT18Document253 pagesFERT18Margarit AnamaryaNo ratings yet
- Synthetic FG Machinery Oil: Trust Omega TODocument2 pagesSynthetic FG Machinery Oil: Trust Omega TOchem KhanNo ratings yet
- Chemistry Signature Assignment PDFDocument3 pagesChemistry Signature Assignment PDFapi-302384998No ratings yet
- Analisis Sensori Produk Stik Sukun (Artocarpus Altilis) Dengan Perlakuan Pendahuluan Blanching Dan Perendaman Dalam Larutan Kalsium KloridaDocument6 pagesAnalisis Sensori Produk Stik Sukun (Artocarpus Altilis) Dengan Perlakuan Pendahuluan Blanching Dan Perendaman Dalam Larutan Kalsium KloridaTommy ChandraNo ratings yet
- Inorganic Chemistry For BiologyDocument163 pagesInorganic Chemistry For BiologyBezabih KeltaNo ratings yet
- Crosby ShacklesDocument1 pageCrosby Shacklesroy sihalohoNo ratings yet
- Chaypy V NotchDocument5 pagesChaypy V NotchGherNo ratings yet
- Flat IronDocument15 pagesFlat IronJane Quenie Caera LibrandaNo ratings yet
- QIAamp Viral RNA Mini HandbookDocument44 pagesQIAamp Viral RNA Mini HandbookYoNo ratings yet
- Isooctyl Mercaptan PDFDocument9 pagesIsooctyl Mercaptan PDFGilar GumelarNo ratings yet
- Pressure Vessel Plates, Alloy Steel, Chromium-Molybdenum-TungstenDocument3 pagesPressure Vessel Plates, Alloy Steel, Chromium-Molybdenum-TungstenMytzy Godoy TapiaNo ratings yet
- Quartz Glued 2Document5 pagesQuartz Glued 2jNo ratings yet
- Astmf 2307-03Document5 pagesAstmf 2307-03Pegfan85No ratings yet
- Supplier+Quality+Manual+V+01 - ChemicalDocument62 pagesSupplier+Quality+Manual+V+01 - ChemicalTrinhTruongNo ratings yet
- 1 s2.0 S2590048X22001030 MainDocument17 pages1 s2.0 S2590048X22001030 MainJeff DatinguinooNo ratings yet
- Form Two ChemistryDocument108 pagesForm Two ChemistryLawrence NgariNo ratings yet
- 4.04 IsophoroneDocument2 pages4.04 Isophoronekhizer iqbalNo ratings yet
- Acquity UPLC H-ClassDocument6 pagesAcquity UPLC H-Classchaerul.anwar554No ratings yet
- Evolution of Gear Quality in Helical PM Gears During ProcessingDocument7 pagesEvolution of Gear Quality in Helical PM Gears During ProcessingyağmurNo ratings yet
- Eucalyptus OilDocument17 pagesEucalyptus OilSorinGeorgeNo ratings yet
- Metal LQPPDocument39 pagesMetal LQPPEddy Laurent OffiNo ratings yet
- UNIT V - Ceramics-Composites-Nano MaterialsDocument59 pagesUNIT V - Ceramics-Composites-Nano MaterialsHarsha MallaNo ratings yet
- Eh PHDocument22 pagesEh PHGörkem Efe100% (1)
- AHD ThesisDocument142 pagesAHD ThesisAkanchhaNo ratings yet
- Ozone: Friend AND Foe: Celebrating ChemistryDocument1 pageOzone: Friend AND Foe: Celebrating ChemistryMarjohn PunzalNo ratings yet
- GasturbinesDocument36 pagesGasturbinesMogili AnjiNo ratings yet