Professional Documents
Culture Documents
Unit I Environmental Pollutant: Dr. Vikesh G. Lade (PH.D.)
Uploaded by
vikeshchemOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Unit I Environmental Pollutant: Dr. Vikesh G. Lade (PH.D.)
Uploaded by
vikeshchemCopyright:
Available Formats
Unit I
Environmental Pollutant
Subject: BTCHE 602T (BCHE)
Environmental Engineering (Theory)
Dr. VIKESH G. LADE (Ph.D.)
Department of Chemical Engineering
Laxminarayan Institute of Technology, Nagpur
Rashtrasant Tukadoji Maharaj Nagpur University, Amravati Road, Nagpur – 440 033
Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License</a>.
Topics of Unit I
Sources & characterization of various pollutants.
Concepts of biodegradability, biosorption,
biomagnifications.
Measurement : COD, BOD, TOD, ThOD, soluble,
suspended, volatile solids, ammonical nitrogen.
Mathematical model for BOD.
Re-oxygenation and de-oxygenation in natural
purification process.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 2
Learning Outcomes
When you have studied this session, you
should be able to:
1. Describe the main types of pollution.
2. Describe the sources of pollution and the way
pollutants reach the environment.
3. Describe the main characteristics of water
pollution
4. Describe the mathematical model for BOD
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 3
Environmental Pollutants
Pollution is defined as the introduction into the
environment of substances liable to cause harm to humans
and other living organisms.
Pollutants may be in the form of gas, liquid, solid or energy.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 4
Pathways of pollution
The pathway of pollution is the way the pollutant moves
from the source, enters into the environment, and finally
how it reaches the human body or other recipient
Once released into the environment, the worst effects of
many pollutants are reduced by one or more of the
following processes:
Dispersion – smoke disperses into the air and is no longer
noticeable away from the source.
Dilution – soluble pollutants are diluted in the water of a river
or lake.
Deposition – some suspended solids carried in a river settle
(are deposited) on the river bed.
Degradation – some substances break down (degrade) by
natural processes into different, simpler substances that are not
polluting.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 5
Wastewater
Sewage or wastewater is a dilute mixture of various wastes from residential,
commercial, industrial and other public places
Inorganic or mineral matter: ash, cinder, sand, grit, mud and other mineral salt
Organic matter: nitrogeneous and nitrogen-free
It is simply that part of the water supply to the community or to the industry which
has been used for different purposes and has been mixed with solids either
suspended or dissolved.
Wastewater is 99.9% water and 0.1% solids. The main task in treating the
wastewater is simply to remove most or all of this 0.1% of solids.
Wastewater contains
organic & inorganic
matters which may be
suspended, colloidal
& dissolved form.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 6
Typical Wastewater Composition
Contaminants Unit Weak Medium Strong
Solids, total (TS) mg/L 350 720 1200
Dissolved, total (TDS) mg/L 250 500 850
Fixed mg/L 145 300 525
Volatile mg/L 105 200 325
Settleable solids (SS) mg/L 100 220 350
Fixed mg/L 20 55 75
Volatile mg/L 80 165 275
Settleable Solids mg/L 5 10 20
BOD5 , 200 C mg/L 110 220 400
Total organic carbon (TOC) mg/L 80 160 290
Chemical oxygen demand (COD) mg/L 250 500 1000
Nitrogen (total as N) mg/L 20 40 85
Organic mg/L 8 15 35
Free ammonia mg/L 12 25 50
Phosphorus (total as P) mg/L 4 8 15
Organic mg/L 1 3 5
Inorganic mg/L 3 5 10
Chlorides mg/L 30 50 100
Sulfate mg/L 20 30 50
Alkalinity (as CaCO3) mg/L 50 100 200
Grease mg/L 50 100 150
Total coliform no/100 ml 106-107 107–108 107–109
Volatile organic compounds (VOCs) mg/L <100 100 -400 > 400
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 7
Effluent Standard for Disposal
Need of study of characteristics of wastewater ?
Information about strength, composition & characteristics of wastewater is
important in the design of treatment system & the amount of pollutants to be
removed up to prescribed level set by the local authority.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 8
Characteristics of Wastewater
The characteristics can be classified as:
1) Physical: i) smell or odour, ii) colour and iii) temperature iv) turbidity v)
solid content
2) Chemical: i) pH, ii) Chloride content, iii) Nitrogen Content, iv) fat, grease
and oil content, v) sulphites, sulphates and H2S gas, vi) dissolved Oxygen
vii) chemical oxygen demand viii) Biochemical oxygen demand
3) Biological characteristics relates to various micro-organism found in
wastewater
Characteristics depends on
1) Source of generation
2) Quality of water used
3) Culture of population
4) Conservation practice
5) Types of industries present
6) Treatment given by industries
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 9
Characteristics & Sources
Characteristic Sources
I Physical Characteristics/ Properties
i) Color Domestic and industrial wastes, natural decay of organic materials
ii) Odor Decomposing wastewater, industrial wastes.
iii) Solids Domestic water supply, domestic and industrial wastes, soil erosion, inflow infiltration
iv) Temperature Domestic and industrial wastes
II Chemical Characteristics
a) Organic:
Carbohydrates Domestic, commercial, and industrial wastes
Fats, oils, and grease Domestic, commercial, and industrial wastes
Pesticides Agricultural wastes
Phenols Industrial wastes
Proteins Domestic, commercial, and industrial wastes
Priority pollutants Domestic, commercial, and industrial wastes
Surfactants Domestic, commercial, and industrial wastes
Volatile organic compounds Domestic, commercial, and industrial wastes
Other Natural decay of organic materials
b) Inorganic:
Alkalinity Domestic wastes, domestic water supply, groundwater infiltration
Chlorides Domestic wastes, domestic water supply, groundwater infiltration
Heavy metals Industrial wastes
Nitrogen Domestic and agricultural wastes
PH Domestic, commercial, and industrial wastes
Phosphorus Domestic, commercial, and industrial wastes natural runoff
Priority polluter Sulfur Domestic water supply; doestic, commercial. And industrial wastes
c) Gases:
Hydrogen sulfide Decomposition of domestic wastes
Methane Decomposition of domestic wastes
Oxygen Domestic water supply , surface-water infiltration
III) Biological constituents:
Animals Open watercourses and treatment plants
Plants Open watercourses and treatment plants
Eubacteria/Archaebacteria Domestic wastes, surface water infiltration, treatment plants .
Viruses Domestic
Dr. Vikesh G.wastes
Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 10
Decomposition of Sewage
Most of the organic matter present in sewage is unstable and
decomposes readily through chemical as well as the biological
processes. The organic matter, which can be decomposed by bacteria
under biological action, is called biodegradable organic matter. Most
of the organic matter present in sewage is biodegradable and hence
undergo biological decomposition, which can be divided into
Aerobic decomposition also called aerobic oxidation.
Anaerobic decomposition also called Putrefactions.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 11
Aerobic Decomposition
Aerobic decomposition is caused by both aerobic bacteria as well as facultative
bacteria operating aerobically, in presence of air or oxygen which is available in the
wastewater in the dissolved form.
These bacteria will then utilize the free oxygen as electron acceptor there by
oxidizing the organic matter to stable and unobjectionable end products.
The stable end products like nitrates, carbon dioxide, sulphates, are formed,
respectively for the three forms of matter, i.e. nitrogenous, carbonaceous, and
sulphurous matter.
Water heat and additional bacteria will also be produced in the biological
oxidation, which can be represented by following equation
COHNS + Bacteria + O2 -------- CO2 + H2O + Bacteria + Energy
The intermediate products formed in the aerobic oxidation of the three types of
organic matter can be known by studying nitrogen, carbon, and sulphur cycles.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 12
Aerobic Decomposition
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 13
Anaerobic Decomposition
If free dissolved oxygen is not available to the sewage, then the anaerobic
decomposition, called putrefaction.
Anaerobic bacteria and facultative bacteria operating anaerobically, will then
flourish and convert the complex organic matter into simpler organic compounds
of nitrogen, carbon, and sulphur.
These anaerobic bacteria survive by extracting and consuming like nitrate and
sulphates. Gases like ammonia, hydrogen sulphide, methane etc. are also evolved
in this decomposition, producing obnoxious (bad) odour.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 14
Anaerobic Decomposition
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 15
Cycles of decomposition
The matter of the universe remains constant, but its form changes
because of biochemical reactions. The complex organic compounds
of biodegradable nature are broken up by biochemical reactions into
simple compounds which are consumed as food by plant and animal
life and the organic matter is formed again. This cycle thus goes on.
From the point of view of sewage treatment, the cycles of
decomposition of the following five elements are of importance:
1. Nitrogen cycle
2. Carbon cycle
3. Sulphur cycle
4. Calcium cycle
5. Phosphorus cycle.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 16
Nitrogen Cycle
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 17
Nitrogen Cycle
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 18
Carbon Cycle
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 19
Carbon Cycle
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 20
Sulphur Cycle
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 21
Sulphur Cycle
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 22
Phosphorus Cycle
The phosphorus cycle relates to the maintenance of level of phosphorus in the soil.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 23
Phosphorus Cycle
The phosphorus cycle relates to the maintenance of level of phosphorus in the soil.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 24
Calcium Cycle
The calcium cycle relates to the maintenance of level of calcium in the soil.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 25
Biomagnification
Biomagnification is the accumulation of a chemical by an organism
from water and food exposure that results in a concentration that is
greater than would have resulted from water exposure only and thus
greater than expected from equilibrium.
In aquatic environments, chemicals that are accumulated through
biomagnification may eventually become toxic to higher organisms as
well.
The lowest substrate concentration that is required to sustain
growth of a species is generally referred to as ‘threshold’
concentration.
In biodegradation, it is the lowest toxic substrate concentration
below which a microorganism cannot degrade the toxic substrate any
further.
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 26
Biomagnification
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 27
Biosorption
Biosorption can be defined as the uptake of organic and inorganic
metal species, both soluble and insoluble, by physicochemical
mechanisms such as adsorption.
In living cells, metabolic activity may also influence this process
because of changes in the physico- chemical characteristics of the
cellular microenvironment.
Almost all biological macromolecules have some affinity for metal
species with cell walls and associated materials being of the greatest
significance in biosorption.
As well as this, cationic species can be accumulated by cells via
transport systems of varying affinity and specificity. Once inside cells,
metal species may be bound, precipitated, localized within
intracellular structures or organelles, or translocated to specific
structures, depending on the element concerned and the organism
Dr. Vikesh G. Lade (Assistant Professor Chemical Engineering, LIT Nagpur) 28
Questions?
For any querry
Dr. Vikesh Gurudas Lade
Mob: 9712499555
email ID: dr.vikeshglade@gmail.com
29
You might also like
- Chapter 1-3 Wastewater TreatmentDocument33 pagesChapter 1-3 Wastewater TreatmentGebrewahid AdhanaNo ratings yet
- Sewage TreatmentDocument17 pagesSewage TreatmentAsim MazumderNo ratings yet
- ME Influent Wastewater Composition November 2020Document1 pageME Influent Wastewater Composition November 2020hadiNo ratings yet
- Biological Wastewater Treatment LectureDocument38 pagesBiological Wastewater Treatment Lectureamin alzuraikiNo ratings yet
- ENVE 309 Fundamentals of Biological Treatment: 2 0 2 1-2022 FA L L Metu EnvironmantalengineeringdepartmentDocument20 pagesENVE 309 Fundamentals of Biological Treatment: 2 0 2 1-2022 FA L L Metu EnvironmantalengineeringdepartmentAslı GözpınarNo ratings yet
- L4. Biological Wastewater Treatment Part 1Document60 pagesL4. Biological Wastewater Treatment Part 1amin alzuraikiNo ratings yet
- Regulation EN-5.0, Water EnvironmentDocument18 pagesRegulation EN-5.0, Water EnvironmentunaismicrobiologyNo ratings yet
- Discharge Standards in Dubai PDFDocument6 pagesDischarge Standards in Dubai PDFHRK65No ratings yet
- Water Treatment Part 1Document81 pagesWater Treatment Part 1Anonymous 8S7URr4F3vNo ratings yet
- CIVL 406: Municipal Wastewater Treatment OverviewDocument137 pagesCIVL 406: Municipal Wastewater Treatment OverviewXuDong GongNo ratings yet
- Unit 5 Water Chemistry 1Document10 pagesUnit 5 Water Chemistry 1shishirNo ratings yet
- Study On Dye Industry Wastewater Treatment Process by Alum and Natural CharcoalDocument5 pagesStudy On Dye Industry Wastewater Treatment Process by Alum and Natural CharcoalInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Research On Effluent-Oxidation PondDocument6 pagesResearch On Effluent-Oxidation PondCHIN FelleNo ratings yet
- Environmental Engg. 3160611 - Lab ManualDocument64 pagesEnvironmental Engg. 3160611 - Lab ManualShreyas PatelNo ratings yet
- Greywater Treatment Using GAC Biofilm Reactor and Sand Filter SystemDocument10 pagesGreywater Treatment Using GAC Biofilm Reactor and Sand Filter SystemkktayNo ratings yet
- TCVN 5945-2005 Industrial Waste Water - Discharge Standard-EnDocument2 pagesTCVN 5945-2005 Industrial Waste Water - Discharge Standard-EnPn ThanhNo ratings yet
- Vietnamese Standard TCVN 5945Document20 pagesVietnamese Standard TCVN 5945titsxuNo ratings yet
- CE 8512 W&WW Analysis LabDocument74 pagesCE 8512 W&WW Analysis LabSuria Prakash50% (2)
- Advances in Textile Wastewater TreatmentDocument17 pagesAdvances in Textile Wastewater TreatmentEswaramoorthi Sellappa Gounder100% (5)
- Anqi 2020 IOP Conf. Ser. Earth Environ. Sci. 565 012038Document5 pagesAnqi 2020 IOP Conf. Ser. Earth Environ. Sci. 565 012038shiwasish swarNo ratings yet
- Trade Effluent Control Regulations 2022Document4 pagesTrade Effluent Control Regulations 2022Ahmed WagihNo ratings yet
- ch4 Water Quality Engineering PDFDocument26 pagesch4 Water Quality Engineering PDFashe zinabNo ratings yet
- Indonesia Project - Confirmation For Foundation Design Condition 140217Document2 pagesIndonesia Project - Confirmation For Foundation Design Condition 140217idilfitriNo ratings yet
- Water 11 01849Document17 pagesWater 11 01849Khaled NaguibNo ratings yet
- Denr Dao 2016-08Document27 pagesDenr Dao 2016-08Patricia Joyce AniNo ratings yet
- Who Standart Water PDFDocument4 pagesWho Standart Water PDFLaily Noor Okvitasari100% (1)
- Advances in Textile Waste Water TreatmentDocument17 pagesAdvances in Textile Waste Water TreatmentYamitakaiNo ratings yet
- A Review On The Application of Nanoporous Zeolite For Sanitary Landfill Leachate TreatmentDocument17 pagesA Review On The Application of Nanoporous Zeolite For Sanitary Landfill Leachate TreatmentEzaira PangalilaNo ratings yet
- Append ADocument12 pagesAppend AK4No ratings yet
- L1-Introduction Environmental EmissionsDocument30 pagesL1-Introduction Environmental EmissionsrushdiNo ratings yet
- Trakhees Waste Water Discharge Quality Standard PDFDocument18 pagesTrakhees Waste Water Discharge Quality Standard PDFWaseem SiddiqueNo ratings yet
- Treatment of Waste Product (WASTEWATERS)Document2 pagesTreatment of Waste Product (WASTEWATERS)aiensyafiqahNo ratings yet
- 3606 8700 1 SMDocument10 pages3606 8700 1 SMputrahidayatulloh860No ratings yet
- Industrial Effluent & Waste ResiduesDocument29 pagesIndustrial Effluent & Waste ResiduesSanjeev NehruNo ratings yet
- CE-311 Lecture On CharacterizationDocument69 pagesCE-311 Lecture On CharacterizationakashNo ratings yet
- Health Minister Regulation on Water Quality ParametersDocument4 pagesHealth Minister Regulation on Water Quality ParametersFajri WidodoNo ratings yet
- Module 5 Water Quality ADocument34 pagesModule 5 Water Quality AAli ZubairNo ratings yet
- Vietnam industrial waste water discharge standardsDocument3 pagesVietnam industrial waste water discharge standardsDung PhanduyNo ratings yet
- DAO 34 and 35 Water Quality Criteria and Effluent RegulationsDocument33 pagesDAO 34 and 35 Water Quality Criteria and Effluent Regulationscris guzonNo ratings yet
- Bhu 202080Document8 pagesBhu 202080anilkumarak9532No ratings yet
- QCVN 08-2008 BTNMT National Technical Regulation On Surface Water QualityDocument9 pagesQCVN 08-2008 BTNMT National Technical Regulation On Surface Water QualityvxzvzxvzvNo ratings yet
- Results & Analysis: Laboratory ReportDocument4 pagesResults & Analysis: Laboratory ReportwaniNo ratings yet
- Desirable Limits Permissible Limit in The Absence of Alternate Source ActualDocument3 pagesDesirable Limits Permissible Limit in The Absence of Alternate Source ActualAnonymous iTzCnMNo ratings yet
- Experiment No. 3 Date: Title: Determination of Chemical Oxygen Demand (COD) in A Given Wastewater SampleDocument10 pagesExperiment No. 3 Date: Title: Determination of Chemical Oxygen Demand (COD) in A Given Wastewater SampleMac357GNo ratings yet
- Removal of Lead (II) From Waste Water by AdsorptionDocument22 pagesRemoval of Lead (II) From Waste Water by AdsorptionAlfonso EncinasNo ratings yet
- 11 - Chapter 5Document23 pages11 - Chapter 5Devendra KhadeNo ratings yet
- Our Drinking Water Quality 2015Document7 pagesOur Drinking Water Quality 2015Shesharam ChouhanNo ratings yet
- Ionizing Radiation As An Efficient AO (R) P Method For Remediation of Waters and WastewatersDocument41 pagesIonizing Radiation As An Efficient AO (R) P Method For Remediation of Waters and WastewatersSajjala SreedharreddyNo ratings yet
- EP917 PowerpointFaDocument80 pagesEP917 PowerpointFaMassiullahNo ratings yet
- Virtual Lab Water Quality 1Document4 pagesVirtual Lab Water Quality 1Maria ComfortNo ratings yet
- Water Quality Standards For Best Designated UsagesDocument7 pagesWater Quality Standards For Best Designated UsagesSagar ApteNo ratings yet
- Characteristic, Analytic and Sampling of WastewaterDocument20 pagesCharacteristic, Analytic and Sampling of WastewaterJack nguyenNo ratings yet
- Chapter Eight 2Document8 pagesChapter Eight 2Hussen MohammedNo ratings yet
- Chapter 01Document31 pagesChapter 01Rushanth ChandraboseNo ratings yet
- The Effect of Sulfate Concentration On Cod RemovalDocument11 pagesThe Effect of Sulfate Concentration On Cod RemovalMuna AzizNo ratings yet
- Key Parameters and Characteristics of Natural Gas Produced Water TablesDocument2 pagesKey Parameters and Characteristics of Natural Gas Produced Water TablesBona UwiragiyeNo ratings yet
- Final Year Project 2015-2016: Treatment of 570 BPD of Produced Water From Oil Fields Through Forward OsmosisDocument12 pagesFinal Year Project 2015-2016: Treatment of 570 BPD of Produced Water From Oil Fields Through Forward Osmosismuhammad junaid ammarNo ratings yet
- Ion Exchange Resins and Adsorbents in Chemical Processing: Second EditionFrom EverandIon Exchange Resins and Adsorbents in Chemical Processing: Second EditionRating: 5 out of 5 stars5/5 (1)
- Multiple Choice Practice Questions/Answers For Online/Omr AITT-2020 2 Year Mech. Ref & Ac. Trade TheoryDocument62 pagesMultiple Choice Practice Questions/Answers For Online/Omr AITT-2020 2 Year Mech. Ref & Ac. Trade TheoryPhi losNo ratings yet
- Biogas HandbookDocument126 pagesBiogas HandbookmoemuneeNo ratings yet
- L4 M4 Learning Principles and Events of Instruction ModifiedDocument10 pagesL4 M4 Learning Principles and Events of Instruction ModifiedvikeshchemNo ratings yet
- Complete Module 72020Document431 pagesComplete Module 72020Josephine TorresNo ratings yet
- L1 Curriculum Analysis 26 8 19Document27 pagesL1 Curriculum Analysis 26 8 19vikeshchemNo ratings yet
- L3 Strategies For Teaching Elements of Content Analysis 26 8 19Document11 pagesL3 Strategies For Teaching Elements of Content Analysis 26 8 19vikeshchemNo ratings yet
- Module 4 Outline PDFDocument12 pagesModule 4 Outline PDFPrasannaNo ratings yet
- Advancement in Drinking Water Treatments From PDFDocument4 pagesAdvancement in Drinking Water Treatments From PDFvikeshchemNo ratings yet
- Advancement in Drinking Water Treatments From PDFDocument4 pagesAdvancement in Drinking Water Treatments From PDFvikeshchemNo ratings yet
- Application of A Solar UV Chlorine Advanced Oxidation Process To Oil Sands Process-Affected Water RemediationDocument10 pagesApplication of A Solar UV Chlorine Advanced Oxidation Process To Oil Sands Process-Affected Water RemediationvikeshchemNo ratings yet
- PaperDocument27 pagesPapervikeshchemNo ratings yet
- 46th Croatian & 6th International Symposium On AgricultureDocument5 pages46th Croatian & 6th International Symposium On AgriculturevikeshchemNo ratings yet
- Guitar Chords:: Chorus: CF CF FCFC CFGC Verse: CF CF FCFC CFGCDocument17 pagesGuitar Chords:: Chorus: CF CF FCFC CFGC Verse: CF CF FCFC CFGCKhuanZumeNo ratings yet
- Jotrun TDSDocument4 pagesJotrun TDSBiju_PottayilNo ratings yet
- Evolution Packet FinalDocument24 pagesEvolution Packet FinalJoaquinNo ratings yet
- C-Dot Max-XlDocument39 pagesC-Dot Max-XlGourav Roy100% (3)
- Digital Water Monitoring and Turbidity Quality System Using MicrocontrollerDocument8 pagesDigital Water Monitoring and Turbidity Quality System Using MicrocontrollerIrin DwiNo ratings yet
- Airs-Lms - Math-10 - q3 - Week 3-4 Module 3 Rhonavi MasangkayDocument19 pagesAirs-Lms - Math-10 - q3 - Week 3-4 Module 3 Rhonavi MasangkayRamil J. Merculio100% (1)
- 5.a Personal Diet Consultant For Healthy MealDocument5 pages5.a Personal Diet Consultant For Healthy MealKishore SahaNo ratings yet
- Solutions: Spheres, Cones and CylindersDocument13 pagesSolutions: Spheres, Cones and CylindersKeri-ann MillarNo ratings yet
- ACL GRC Risk Manager - Usage Guide V1.1Document28 pagesACL GRC Risk Manager - Usage Guide V1.1Rohit ShettyNo ratings yet
- Internal Resistance and Matching in Voltage SourceDocument8 pagesInternal Resistance and Matching in Voltage SourceAsif Rasheed Rajput100% (1)
- Telecommunications TechnicianDocument4 pagesTelecommunications Technicianapi-78381064No ratings yet
- Organic Facial Remedies Versus Inorganic Facial RemediesDocument13 pagesOrganic Facial Remedies Versus Inorganic Facial Remediesapi-271179911No ratings yet
- Laws of ThermocoupleDocument3 pagesLaws of ThermocoupleMourougapragash SubramanianNo ratings yet
- Pharma TestDocument2 pagesPharma TestMuhammad AdilNo ratings yet
- TNG UPDATE InstructionsDocument10 pagesTNG UPDATE InstructionsDiogo Alexandre Crivelari CrivelNo ratings yet
- SolarBright MaxBreeze Solar Roof Fan Brochure Web 1022Document4 pagesSolarBright MaxBreeze Solar Roof Fan Brochure Web 1022kewiso7811No ratings yet
- A ULTIMA ReleaseNotesAxiomV PDFDocument38 pagesA ULTIMA ReleaseNotesAxiomV PDFIVANALTAMARNo ratings yet
- Boala Cronica Obstructive: BpocDocument21 pagesBoala Cronica Obstructive: BpocNicoleta IliescuNo ratings yet
- Telephone Triggered SwitchesDocument22 pagesTelephone Triggered SwitchesSuresh Shah100% (1)
- Galambos 1986Document18 pagesGalambos 1986gcoNo ratings yet
- Kltdensito2 PDFDocument6 pagesKltdensito2 PDFPutuWijayaKusumaNo ratings yet
- Ek Pardesi Mera Dil Le Gaya Lyrics English Translation - Lyrics GemDocument1 pageEk Pardesi Mera Dil Le Gaya Lyrics English Translation - Lyrics Gemmahsa.molaiepanahNo ratings yet
- 02 MortarsDocument2 pages02 MortarsTarun kumar DigarseNo ratings yet
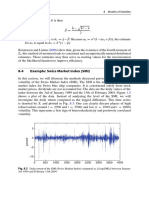
- 8.4 Example: Swiss Market Index (SMI) : 188 8 Models of VolatilityDocument3 pages8.4 Example: Swiss Market Index (SMI) : 188 8 Models of VolatilityNickesh ShahNo ratings yet
- Document-SAP EWM For Fashion 1.0: 1.general IntroductionDocument3 pagesDocument-SAP EWM For Fashion 1.0: 1.general IntroductionAnonymous u3PhTjWZRNo ratings yet
- Useful Relations in Quantum Field TheoryDocument30 pagesUseful Relations in Quantum Field TheoryDanielGutierrez100% (1)
- Business StrategiesDocument2 pagesBusiness Strategiesthristanlexter694No ratings yet
- Stellar Structure and EvolutionDocument222 pagesStellar Structure and Evolutionjano71100% (2)
- Eole Press KitDocument15 pagesEole Press KitBob AndrepontNo ratings yet
- GD&T WIZ Tutor Covers The Vast Breadth of Geometric Dimensioning and Tolerancing Without Compromising On The Depth. The Topics Covered AreDocument1 pageGD&T WIZ Tutor Covers The Vast Breadth of Geometric Dimensioning and Tolerancing Without Compromising On The Depth. The Topics Covered AreVinay ManjuNo ratings yet